Cu Nanoparticle-Loaded Nanovesicles with Antibiofilm Properties. Part I: Synthesis of New Hybrid Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CuNPs
2.3. Characterization of CuNPs
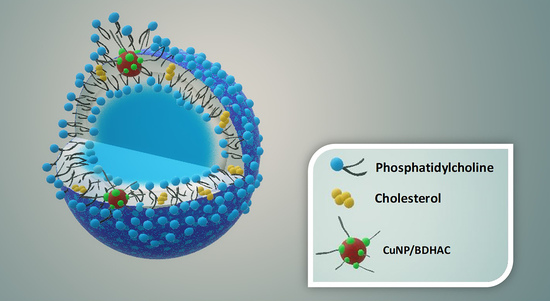
2.4. Preparation of Empty NVs and NP-Loaded NVs
2.5. Purification of NP-Loaded NVs
2.6. Characterization of Empty NVs and NP-Loaded NVs
3. Results
3.1. Synthesis and Morphological Characterization of CuNPs
3.2. Preparation and Characterization of Empty NVs
3.3. Preparation and Characterization of NP-Loaded NVs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, A.W. Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N.; Inhibiting, P. fluorescens biofilms with fluoropolymer-embedded silver nanoparticles: An in-situ spectroscopic study. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Chari, N.; Felix, L.O.; Davoodbasha, M.A.; Sulaiman Ali, A.; Nooruddin, T. In vitro and in vivo antibiofilm effect of copper nanoparticles against aquaculture pathogens. Biocatal. Agric. Biotechnol. 2017, 10, 336–341. [Google Scholar] [CrossRef]
- Galli, R.; Hall, M.C.; Breitenbach, E.R.; Colpani, G.L.; Zanetti, M.; de Mello, J.M.M.; Silva, L.L.; Fiori, M.A. Antibacterial polyethylene-ethylene vinyl acetate polymeric blend by incorporation of zinc oxide nanoparticles. Polym. Test. 2020, 89, 106554. [Google Scholar] [CrossRef]
- Eltz, F.Z.; Vebber, M.C.; Aguzzoli, C.; Machado, G.; Da Silva Crespo, J.; Giovanela, M. Preparation, characterization and application of polymeric thin films containing silver and copper nanoparticles with bactericidal activity. J. Environ. Chem. Eng. 2020, 8, 103745. [Google Scholar] [CrossRef]
- Khan, Z.; Al-Thabaiti, S.A. Biogenic silver nanoparticles: Green synthesis, encapsulation, thermal stability and antimicrobial activities. J. Mol. Liq. 2019, 289, 111102. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Wu, M.; Jia, H. Preparation and antibacterial activities of copper nanoparticles encapsulated by carbon. New Carbon Mater. 2019, 34, 382–389. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar] [CrossRef] [PubMed]
- Beeton, M.L.; Aldrich-Wright, J.R.; Bolhuis, A. The antimicrobial and antibiofilm activities of copper(II) complexes. J. Inorg. Biochem. 2014, 140, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering copper nanoparticles synthesized on the surface of carbon nanotubes for anti-microbial and anti-biofilm applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf. B Biointerfaces 2006, 48, 112–118. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Sabbatini, L.; Zambonin, P.G.; Tantillo, G.; Ghibelli, L.; D’Alessio, M.; Bleve-Zacheo, T.; Traversa, E. Antifungal activity of polymer-based copper nanocomposite coatings. Appl. Phys. Lett. 2004, 85. [Google Scholar] [CrossRef]
- Weir, E.; Lawlor, A.; Whelan, A.; Regan, F. The use of nanoparticles in anti-microbial materials and their characterization. Analyst 2008, 133, 835–845. [Google Scholar] [CrossRef]
- Anyaogu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C 2016, 60, 195–203. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M.; Manikandan, E.; Kumar, S.S.; Pugazhendhi, A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B Biol. 2018, 188, 126–134. [Google Scholar] [CrossRef]
- Cioffi, N.; Ditaranto, N.; Torsi, L.; Picca, R.A.; De Giglio, E.; Sabbatini, L.; Novello, L.; Tantillo, G.; Bleve-Zacheo, T.; Zambonin, P.G. Synthesis, analytical characterization and bioactivity of Ag and Cu nanoparticles embedded in poly-vinyl-methyl-ketone films. Anal. Bioanal. Chem. 2005, 382, 1912–1918. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Alviña, R.; Zegarra, K.; Pérez, B.; Pozo, P. Antibacterial activities of copper nanoparticles in hybrid microspheres. J. Nanosci. Nanotechnol. 2019, 19, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Quaiser, S.A. A New method for the preparation of nanostructured metal clusters. Angew. Chemie Int. Ed. English 1995, 34, 2240–2241. [Google Scholar] [CrossRef]
- Cioffi, N.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Zambonin, P.G. Nanomaterials for controlled metal release and process for their production. European Patent Application EP 2123797 B1, 12 August 2015. [Google Scholar]
- Cioffi, N.; Ditaranto, N.; Sabbatini, L.; Tantillo, G.; Torsi, L.; Zambonin, P.G. Bioactive Metal Nanomaterials Stabilized by Bioactive Agents and Preparation Process. European Patent Application EP 2157211 B9, 2 March 2016. [Google Scholar]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Estupiñán, O.R.; Blanco-López, M.C.; Pazos, C. Using factorial experimental design to prepare size-tuned nanovesicles. Ind. Eng. Chem. Res. 2016, 55, 9164–9175. [Google Scholar] [CrossRef] [Green Version]
- Pando, D.; Gutiérrez, G.; Coca, J.; Pazos, C. Preparation and characterization of niosomes containing resveratrol. J. Food Eng. 2013, 117, 227–234. [Google Scholar] [CrossRef]
- Liu, K.; Li, H.; Williams, G.R.; Wu, J.; Zhu, L.M. pH-responsive liposomes self-assembled from electrosprayed microparticles, and their drug release properties. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 537, 20–27. [Google Scholar] [CrossRef]
- Et-Thakafy, O.; Delorme, N.; Gaillard, C.; Mériadec, C.; Artzner, F.; Lopez, C.; Guyomarch, F. Mechanical properties of membranes composed of gel-phase or fluid-phase phospholipids probed on liposomes by atomic force spectroscopy. Langmuir 2017, 33, 5117–5126. [Google Scholar] [CrossRef]
- Van Swaay, D.; Demello, A. Microfluidic methods for forming liposomes. Lab Chip 2013, 13, 752–767. [Google Scholar] [CrossRef]
- Montefusco-Pereira, C.V.; Formicola, B.; Goes, A.; Re, F.; Marrano, C.A.; Mantegazza, F.; Carvalho-Wodarz, C.; Fuhrmann, G.; Caneva, E.; Nicotra, F.; et al. Coupling quaternary ammonium surfactants to the surface of liposomes improves both antibacterial efficacy and host cell biocompatibility. Eur. J. Pharm. Biopharm. 2020, 149, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225. [Google Scholar] [CrossRef]
- Ardizzone, A.; Blasi, D.; Vona, D.; Rosspeintner, A.; Punzi, A.; Altamura, E.; Grimaldi, N.; Sala, S.; Vauthey, E.; Farinola, G.M.; et al. Highly stable and red-emitting nanovesicles incorporating lipophilic diketopyrrolopyrroles for cell imaging. Chem. A Eur. J. 2018, 24, 11386–11392. [Google Scholar] [CrossRef] [Green Version]
- Pando, D.; Matos, M.; Gutiérrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf. B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Marchianò, V.; Matos, M.; Serrano-Pertierra, E.; Gutiérrez, G.; Blanco-López, M.C. Vesicles as antibiotic carrier: State of art. Int. J. Pharm. 2020, 585, 119478. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Oh, S.G.; Mun, J.Y.; Han, S.S. Effects of silver nanoparticles on the fluidity of bilayer in phospholipid liposome. Colloids Surf. B Biointerfaces 2005, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.A.M.; Azzazy, H.M.E. Sustained broad-spectrum antibacterial effects of nanoliposomes loaded with silver nanoparticles. Nanomedicine 2014, 9, 1301–1310. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W. Size-selective synthesis of nanostructured transition metal clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- García-Manrique, P.; Machado, N.D.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Effect of drug molecular weight on niosomes size and encapsulation efficiency. Colloids Surf. B Biointerfaces 2020, 186, 110711. [Google Scholar] [CrossRef]
- Bothun, G.D. Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol. 2008, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sportelli, M.C.; Longano, D.; Bonerba, E.; Tantillo, G.; Torsi, L.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Electrochemical preparation of synergistic nanoantimicrobials. Molecules 2020, 25, 49. [Google Scholar] [CrossRef] [Green Version]
- Varona, S.; Martín, Á.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- Tsumoto, K.; Matsuo, H.; Tomita, M.; Yoshimura, T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids Surf. B Biointerfaces 2009, 68, 98–105. [Google Scholar] [CrossRef]
- Becher, P. Emulsions Theory and Practice, 2nd ed.; Reinhold Publ. Corp. Chapman & Hall: New York, NY, USA, 1965. [Google Scholar]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Schulz, M.; Olubummo, A.; Binder, W.H. Beyond the lipid-bilayer: Interaction of polymers and nanoparticles with membranes. Soft Matter 2012, 8, 4849–4864. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Boros, B.V.; Ostafe, V. Evaluation of ecotoxicology assessment methods of nanomaterials and their effects. Nanomaterials 2020, 10, 610. [Google Scholar] [CrossRef] [Green Version]







| TDoAC (Cu@TDoAC) | ||||||||||||
| Membrane Components | Solvents | Evaporation | Hydration PATM | |||||||||
| PC:CHO Molar Ratio | Lipid (mM) | LIP:CuNPs (w/w) | CHCl3:THF (v/v) | Vol (mL) | T °C | P (mbar) | R (rpm) | t (min) | T °C | R (rpm) | t (min) | |
| Test1 | 1:0 | 10 | 500:1 | 9:1 | 20 | 41 | 140 | 140 | 40 | 60 | 150 | 20 |
| Test2 | 2:1 | 10 | 700:1 | 9:1 | 30 | 50 | 490 | 150 | 60 | 60 | 150 | 20 |
| Test3 | 2:1 | 10 | 1500:1 | 9:1 | 15 | 52 | 180 | 150 | 90 | 55 | 150 | 30 |
| BDHAC (Cu@BDHAC) | ||||||||||||
| Membrane Components | Solvents | Evaporation | Hydration PATM | |||||||||
| PC:CHO Molar Ratio | Lipid (mM) | LIP:CuNPs (w/w) | CHCl3:THF (v/v) | Vol (mL) | T °C | P (mbar) | R (rpm) | t (min) | T °C | R (rpm) | t (min) | |
| Test1 | 2:1 | 5 | 2000:1 | 9:1 | 25 | 53 | 150 | 150 | 60 | 50 | 150 | 5 |
| Test2 | 2:1 | 5 | 1200:1 | 9:1 | 25 | 53 | 150 | 150 | 90 | 50 | 150 | 5 |
| Test3 | 2:1 | 5 | 800:1 | 9:1 | 25 | 53 | 150 | 150 | 30 | 50 | 150 | 5 |
| SAMPLE | LIP:NPs (w/w) | ζ-Potential (mV) |
|---|---|---|
| NVs | -- | −24 ± 5 |
| Hybrid 1 | 2000:1 | −11 ± 4 |
| Hybrid 2 | 1200:1 | 5 ± 4 |
| Hybrid 3 | 800:1 | 21 ± 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarcina, L.; García-Manrique, P.; Gutiérrez, G.; Ditaranto, N.; Cioffi, N.; Matos, M.; Blanco-López, M.d.C. Cu Nanoparticle-Loaded Nanovesicles with Antibiofilm Properties. Part I: Synthesis of New Hybrid Nanostructures. Nanomaterials 2020, 10, 1542. https://doi.org/10.3390/nano10081542
Sarcina L, García-Manrique P, Gutiérrez G, Ditaranto N, Cioffi N, Matos M, Blanco-López MdC. Cu Nanoparticle-Loaded Nanovesicles with Antibiofilm Properties. Part I: Synthesis of New Hybrid Nanostructures. Nanomaterials. 2020; 10(8):1542. https://doi.org/10.3390/nano10081542
Chicago/Turabian StyleSarcina, Lucia, Pablo García-Manrique, Gemma Gutiérrez, Nicoletta Ditaranto, Nicola Cioffi, Maria Matos, and Maria del Carmen Blanco-López. 2020. "Cu Nanoparticle-Loaded Nanovesicles with Antibiofilm Properties. Part I: Synthesis of New Hybrid Nanostructures" Nanomaterials 10, no. 8: 1542. https://doi.org/10.3390/nano10081542