Laser-Ablative Synthesis of Stable Aqueous Solutions of Elemental Bismuth Nanoparticles for Multimodal Theranostic Applications
Abstract
:1. Introduction
2. Materials and Methods
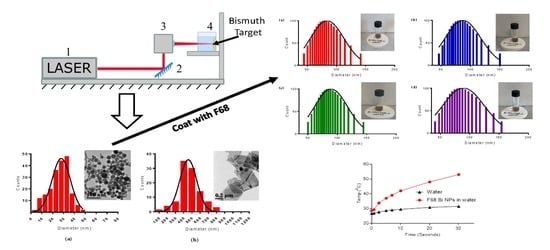
2.1. Synthesis of Nanoparticles
2.2. Surface Modification of Bi NPs
2.3. Characterization of Nanoparticles
2.4. Photothermal Gradient Measurement
3. Results and Discussion
3.1. Physical Characterization
3.2. Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; He, F.; Dong, Y.; Yang, D.; Dai, Y.; Xu, L.; Lv, R.; Gai, S.; Yang, P.; Lin, J. Bismuth Nanoparticles with “Light” Property Served as a Multifunctional Probe for X-ray Computed Tomography and Fluorescence Imaging. Chem. Mater. 2018, 30, 3301–3307. [Google Scholar] [CrossRef]
- Yu, X.; Li, A.; Zhao, C.; Yang, K.; Chen, X.; Li, W. Ultrasmall Semimetal Nanoparticles of Bismuth for Dual-Modal Computed Tomography/Photoacoustic Imaging and Synergistic Thermoradiotherapy. ACS Nano 2017, 11, 3990–4001. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- De La Vega, J.C.; Häfeli, U.O. Utilization of nanoparticles as X-ray contrast agents for diagnostic imaging applications. Contrast Media Mol. Imaging 2015, 10, 81–95. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Chen, X.; Li, S.; Han, Q.; Li, L.; Wang, C. Biomolecules-assisted synthesis of degradable bismuth nanoparticles for dual-modal imaging-guided chemo-photothermal therapy. Chem. Eng. J. 2020, 382, 122720. [Google Scholar] [CrossRef]
- Bierer, D.W. Bismuth Subsalicylate: History, Chemistry, and Safety. Rev. Infect. Dis. 1990, 12, S3–S8. [Google Scholar] [CrossRef]
- Wang, F.; Tang, R.; Yu, H.; Gibbons, P.C.; Buhro, W.E. Size- and Shape-Controlled Synthesis of Bismuth Nanoparticles. Chem. Mater. 2008, 20, 3656–3662. [Google Scholar] [CrossRef]
- Carotenuto, G.; Hison, C.L.; Capezzuto, F.; Palomba, M.; Perlo, P.; Conte, P. Synthesis and thermoelectric characterisation of bismuth nanoparticles. J. Nanoparticle Res. 2008, 11, 1729. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, X.; Zhang, C.; Jiang, Y.; Fu, Y.-Y.; Yu, C.; Sun, S.-K.; Yan, X.-P. Facile Synthesis of Uniform-Sized Bismuth Nanoparticles for CT Visualization of Gastrointestinal Tract in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 12720–12726. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Delaporte, P.; Pereira, A.; Grojo, D.; Torres, R.; Sarnet, T.; Sentis, M. Nanofabrication with Pulsed Lasers. Nanoscale Res. Lett. 2010, 5, 454. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Fojtik, A.; Henglein, A. Laser ablation of films and suspended particles in a solvent: Formation of cluster and colloid solutions. Ber. Bunsen-Ges. 1993, 97, 252–254. [Google Scholar]
- Mafuné, F.; Kohno, J.-Y.; Takeda, Y.; Kondow, T.; Sawabe, H. Formation of Gold Nanoparticles by Laser Ablation in Aqueous Solution of Surfactant. J. Phys. Chem. B 2001, 105, 5114–5120. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Synthesis of colloidal nanoparticles during femtosecond laser ablation of gold in water. J. Appl. Phys. 2003, 94, 7941–7943. [Google Scholar] [CrossRef] [Green Version]
- Hebié, S.; Holade, Y.; Maximova, K.; Sentis, M.; Delaporte, P.; Kokoh, K.B.; Napporn, T.W.; Kabashin, A.V. Advanced Electrocatalysts on the Basis of Bare Au Nanomaterials for Biofuel Cell Applications. ACS Catal. 2015, 5, 6489–6496. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [Green Version]
- Baati, T.; Al-Kattan, A.; Esteve, M.-A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: In vivo assessment of safety and biodistribution. Sci. Rep. 2016, 6, 25400. [Google Scholar] [CrossRef] [Green Version]
- Kabashin, A.V.; Singh, A.; Swihart, M.T.; Zavestovskaya, I.N.; Prasad, P.N. Laser-Processed Nanosilicon: A Multifunctional Nanomaterial for Energy and Healthcare. ACS Nano 2019, 13, 9841–9867. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser- synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova-Kuznetsova, E.; Tikhonowski, G.; Popov, A.A.; Duflot, V.; Deyev, S.; Klimentov, S.; Zavestovskaya, I.; Prasad, P.N.; Kabashin, A.V. Laser-Ablative Synthesis of Isotope-Enriched Samarium Oxide Nanoparticles for Nuclear Nanomedicine. Nanomaterials 2019, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondal, M.A.; Saleh, T.A.; Drmosh, Q. Optical Properties of Bismuth Oxide Nanoparticles Synthesized by Pulsed Laser Ablation in Liquids. Sci. Adv. Mater. 2012, 4, 507–510. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Velarde Granados, E.; Solís-Casados, D.A.; Olea-Mejía, O.; Espinosa-Pesqueira, M.; Haro-Poniatowski, E. Preparation of bismuth-based nanosheets by ultrasound-assisted liquid laser ablation. Appl. Phys. A 2016, 122, 433. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari H, H. Formation, gradual oxidation mechanism and tunable optical properties of Bi/Bi2O3 nanoparticles prepared by Nd:YAG laser ablation in liquid: Dissolved oxygen as genesis of tractable oxidation. Mater. Res. Bull. 2018, 97, 421–427. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Optical and structural properties of Bi-based nanoparticles prepared via pulsed Nd:YAG laser ablation in organic liquids. Appl. Phys. A 2018, 124, 406. [Google Scholar] [CrossRef]
- Dadashi, S.; Delavari, H.; Poursalehi, R. Optical Properties and Colloidal Stability Mechanism of Bismuth Nanoparticles Prepared by Q-switched Nd:Yag Laser Ablation in Liquid. Procedia Mater. Sci. 2015, 11, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dixit, C.K. 3-Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–58. [Google Scholar] [CrossRef]
- Huang, H.; Tian, N.; Jin, S.; Zhang, Y.; Wang, S. Syntheses, characterization and nonlinear optical properties of a bismuth subcarbonate Bi2O2CO3. Solid State Sci. 2014, 30, 1–5. [Google Scholar] [CrossRef]
- Dong, F.; Zheng, A.; Sun, Y.; Fu, M.; Jiang, B.; Ho, W.-K.; Lee, S.C.; Wu, Z. One-pot template-free synthesis, growth mechanism and enhanced photocatalytic activity of monodisperse (BiO)2CO3 hierarchical hollow microspheres self-assembled with single-crystalline nanosheets. CrystEngComm 2012, 14, 3534–3544. [Google Scholar] [CrossRef]
- Marinho, J.Z.; Santos, L.M.; Macario, L.R.; Longo, E.; Machado, A.E.H.; Patrocinio, A.O.T.; Lima, R.C. Rapid Preparation of (BiO)2CO3 Nanosheets by Microwave-Assisted Hydrothermal Method with Promising Photocatalytic Activity Under UV-Vis Light. J. Braz. Chem. Soc. 2015, 26, 498–505. [Google Scholar]
- Xiong, T.; Huang, H.; Sun, Y.; Dong, F. In situ synthesis of a C-doped (BiO)2CO3 hierarchical self-assembly effectively promoting visible light photocatalysis. J. Mater. Chem. A 2015, 3, 6118–6127. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. The molecular structure of bismuth oxide by Raman spectroscopy. J. Solid State Chem. 1992, 97, 319–331. [Google Scholar] [CrossRef]
- Lewis, J.S.a.R. In situ micro-Raman studies of laser-induced bismuth oxidation reveals metastability of β-Bi2O3 microislands. Opt. Mater. Express 2014, 4, 2133–2144. [Google Scholar]
- Taylor, P.; Sunder, S.; Lopata, V.J. Structure, spectra and stability of solid bismuth carbonates. Can. J. Chem. 1984, 62, 2863–2873. [Google Scholar] [CrossRef]
- Wang, Y.W.; Hong, B.H.; Kim, K.S. Size Control of Semimetal Bismuth Nanoparticles and the UV−Visible and IR Absorption Spectra. J. Phys. Chem. B 2005, 109, 7067–7072. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, C.; Huang, R.; Peng, H.; Tang, X. Investigation of Optical and Photocatalytic Properties of Bismuth Nanospheres Prepared by a Facile Thermolysis Method. J. Phys. Chem. C 2014, 118, 1155–1160. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Q.; Cao, J.-j.; Huang, R.-j.; Ho, W.; Lee, S.C. In situ Fabrication of α-Bi2O3/(BiO)2CO3 Nanoplate Heterojunctions with Tunable Optical Property and Photocatalytic Activity. Sci. Rep. 2016, 6, 23435. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Li, Q.; Sun, Y.; Ho, W.-K. Noble Metal-Like Behavior of Plasmonic Bi Particles as a Cocatalyst Deposited on (BiO)2CO3 Microspheres for Efficient Visible Light Photocatalysis. ACS Catal. 2014, 4, 4341–4350. [Google Scholar] [CrossRef]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulmahn, J.C.; Tikhonowski, G.; Popov, A.A.; Kuzmin, A.; Klimentov, S.M.; Kabashin, A.V.; Prasad, P.N. Laser-Ablative Synthesis of Stable Aqueous Solutions of Elemental Bismuth Nanoparticles for Multimodal Theranostic Applications. Nanomaterials 2020, 10, 1463. https://doi.org/10.3390/nano10081463
Bulmahn JC, Tikhonowski G, Popov AA, Kuzmin A, Klimentov SM, Kabashin AV, Prasad PN. Laser-Ablative Synthesis of Stable Aqueous Solutions of Elemental Bismuth Nanoparticles for Multimodal Theranostic Applications. Nanomaterials. 2020; 10(8):1463. https://doi.org/10.3390/nano10081463
Chicago/Turabian StyleBulmahn, Julia C., Gleb Tikhonowski, Anton A. Popov, Andrey Kuzmin, Sergey M. Klimentov, Andrei V. Kabashin, and Paras N. Prasad. 2020. "Laser-Ablative Synthesis of Stable Aqueous Solutions of Elemental Bismuth Nanoparticles for Multimodal Theranostic Applications" Nanomaterials 10, no. 8: 1463. https://doi.org/10.3390/nano10081463