An Investigative Study on the Effect of Pre-Coating Polymer Solutions on the Fabrication of Low Cost Anti-Adhesive Release Paper
Abstract
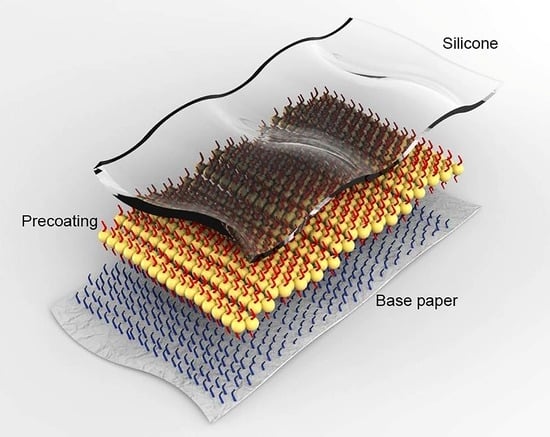
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Pre-coating Solutions
- PS was synthesized according to the procedure described by Keller et al. [20]. Monodispersed PS was synthesized by emulsifier-free emulsion polymerization of styrene using a modified procedure by Fang et al. [21]. In a typical synthesis, deionized water (150 mL) was placed in a 250 mL flask, followed by styrene (7.7 g), KPS (0.06 g), and NaCl. The synthesis was conducted at constant stirring (800 rpm) by magnetic stirrer, and the temperature of water bath was maintained at 80 °C. Post synthesis, the solution was evaporated under vacuum leaving behind the polymer particles. The solution of Styrene dispersion was then diluted by deionized water to final concentrations ranging from 4 to 0.1% (with step 0.5%). Nine concentrations were produced in total.
- PEVA emulsion was diluted with deionized water to final concentrations ranging from 9.5 to 0.5% of PEVA. There were 11 concentrations in total.
- The CC powder was placed in a beaker, and water was added to bring the final concentrations to between 0.25 and 2% (with steps of 0.25%). The solution was heated at 75 °C for 45 min with overhead stirring at 250 rpm.
- PVOH was diluted by deionized water to concentrations ranging from 9.5 to 2% (with steps of 0.5%). Solutions were stirred by magnetic stirrer while heating to 90 °C for 90 min. Eleven concentrations were produced in total.
2.2.2. Paper Coating
2.2.3. Atomic Force Microscopy
2.2.4. Scanning Electron Microscopy
2.2.5. Raman spectroscopy
2.2.6. Contact Angle Measurements
2.2.7. Adhesive Tape Peel Test
2.2.8. Ink Drop Test
3. Results and Discussion
3.1. Optimal Pre-coating Blends Concentrations
3.2. Surface Study
3.3. Adhesive Tape Peel Test and Ink Drop Test
3.4. Raman Spectroscopy
3.5. Precoater Blends Comparison
3.6. Cost Reduction Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuo, A.C.M. Silicone Release Coatings for the Pressure Sensitive Industry—Overview and Trends; DC Corporation: Barry, UK, 2003; pp. 1–4. [Google Scholar]
- Kinning, D.J.; Schneider, H.M. Release coatings for pressure sensitive adhesives. In Adhesion Science and Engineering; Elsevier BV: Amsterdam, The Netherlands, 2002; pp. 535–571. [Google Scholar]
- Horgnies, M.; Darque-Ceretti, E.; Felder, E. Relationship between the fracture energy and the mechanical behaviour of pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2007, 27, 661–668. [Google Scholar] [CrossRef]
- Paltakari, J.; Lehtinen, E.; Imppola, O. Introduction to pigment coating and surface sizing of paper and board. In Papermaking Science and Technology, Pigment Coating and Surface Sizing of Paper, Book 11, 2nd ed.; Paltakari, J., Ed.; Paper Engineers’ Association: Helsinki, Finland, 2009; pp. 12–28. [Google Scholar]
- Jones, L.A.; Schmidt, R.G. Silicone release coating technology. In Technology of Pressure-Sensitive Adhesives and Products; Informa UK Limited: London, UK, 2008; Volume 2, pp. 9:1–9:29. [Google Scholar]
- Clint, J.H. Adhesion and components of solid surface energies. Curr. Opin. Colloid Interface Sci. 2001, 6, 28–33. [Google Scholar] [CrossRef]
- Kinning, D.J. Surface and interfacial structure of release coatings for pressure sensitive adhesives I. polyvinyl N-Alkyl carbamates. J. Adhes. 1997, 60, 249–274. [Google Scholar] [CrossRef]
- Chibowski, E.; Perea-Carpio, R. Problems of contact angle and solid surface free energy determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Ghasemi, D. Rheological and adhesion properties of acrylic pressure-sensitive adhesives. J. Appl. Polym. Sci. 2010, 120, 411–418. [Google Scholar] [CrossRef]
- Kowalski, A.; Czech, Z. The effects of substrate surface properties on tack performance of acrylic Pressure-Sensitive Adhesives (PSAs). Int. J. Adhes. Adhes. 2015, 60, 9–15. [Google Scholar] [CrossRef]
- Finley, J.H. Spectrophotometric determination of polyvinyl alcohol in paper coatings. Anal. Chem. 1961, 33, 1925–1927. [Google Scholar] [CrossRef]
- Hu, Z.; Zen, X.; Gong, J.; Deng, Y. Water resistance improvement of paper by superhydrophobic modification with microsized CaCO3 and fatty acid coating. Colloids Surf. A Physicochem. Eng. Asp. 2009, 351, 65–70. [Google Scholar] [CrossRef]
- Gaeta, A.C.; Swei, G.S. Waterproof Paper-Backed Coated Abrasives. U.S. Patent No. 5,624,471, 29 April 1997. [Google Scholar]
- George, R.A. Manufacture of Glassine Papers. U.S. Patent No. 1921540, 8 August 1933. [Google Scholar]
- Thomas, P.S.; Maguire, T.F. Siloxane Paper Release Coatings. U.S. Patent No. 3385727, 28 May 1964. [Google Scholar]
- Schuman, T.; Wikström, M.; Rigdahl, M. Coating of surface-modified papers with poly(vinyl alcohol). Surf. Coat. Technol. 2004, 183, 96–105. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ayoub, A.; Daystar, J.S.; Venditti, R.A.; Pawlak, J.J. Enhanced absorbent products incorporating cellulose and its derivatives: A review. BioResources 2013, 8, 6556–6629. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.T.; Tsai, R.-S.; Lee, Y.-D. Preparation of block copolyetheramides and their properties as hot melt adhesives. J. Appl. Polym. Sci. 1989, 37, 1863–1876. [Google Scholar] [CrossRef]
- Barrueso-Martínez, M.L.; Ferrándiz-Gómez, T.D.P.; Romero-Sánchez, M.D.; Martín-Martínez, J.M. Characterization of eva-based adhesives containing different amounts of rosin ester or polyterpene tackifier. J. Adhes. 2003, 79, 805–824. [Google Scholar] [CrossRef]
- Keller, K.; Yakovlev, A.; Grachova, E.V.; Vinogradov, A.V. Inkjet printing of multicolor daylight visible opal holography. Adv. Funct. Mater. 2018, 28, 1706903. [Google Scholar] [CrossRef]
- Fang, J.; Xuan, Y.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Ser. E Technol. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- ISO. International Standard ISO 29862:2007: Self Adhesive Tapes—Determination of Peel Adhesion Properties; ISO: London, UK, 2007. [Google Scholar]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Sanoria, A.; Ulbricht, D.; Brüll, R.; Schuster, T. Monitoring crosslinking inhomogeneities in ethylene vinyl acetate photovoltaic encapsulants using Raman microscopy. RSC Adv. 2015, 5, 93522–93529. [Google Scholar] [CrossRef]
- Petrou, M.; Edwards, H.G.M.; Janaway, R.C.; Thompson, G.B.; Wilson, A.S. Fourier-transform Raman spectroscopic study of a Neolithic waterlogged wood assemblage. Anal. Bioanal. Chem. 2009, 395, 2131–2138. [Google Scholar] [CrossRef] [Green Version]
- Chernev, B.; Hirschl, C.; Eder, G.C. Non-destructive determination of ethylene vinyl acetate cross-linking in photovoltaic (PV) modules by raman spectroscopy. Appl. Spectrosc. 2013, 67, 1296–1301. [Google Scholar] [CrossRef]
- Schenzel, K.; Fischer, S. NIR FT Raman spectroscopy–A rapid analytical tool for detecting the transformation of cellulose polymorphs. Cellul. 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Sánchez-Márquez, J.A.; Fuentes-Ramírez, R.; Cano-Rodriguez, I.; Gamiño-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane made of cellulose acetate with polyacrylic acid reinforced with carbon nanotubes and its applicability for chromium removal. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, R.; Ohno, T.; Kaneyasu, J.F.; Leproux, P.; Couderc, V.; Kita, H.; Kano, H. Dynamical study of the water penetration process into a cellulose acetate film studied by coherent anti-Stokes Raman scattering (CARS) microspectroscopy. Chem. Phys. Lett. 2016, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Mamusa, M.; Tempesti, P.; Bartolini, A.; Carretti, E.; Ghobadi, A.F.; Smets, J.; Aouad, Y.G.; Baglioni, P. Associative properties of poly(ethylene glycol)–poly(vinyl acetate) comb-like graft copolymers in water. Nanoscale 2019, 11, 6635–6643. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yarbrough, J.M.; Mittal, A.; Tucker, M.; Vinzant, T.B.; Decker, S.R.; Himmel, M.E. In situ label-free imaging of hemicellulose in plant cell walls using stimulated Raman scattering microscopy. Biotechnol. Biofuels 2016, 9, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yoo, C.G.; Yu, C.X.; Kim, T.H. Raman spectroscopic characterization of photonanocatalyst aided alkaline pretreated corn stover biomass. Adv. Mater. Res. 2014, 875, 1576–1580. [Google Scholar] [CrossRef]
- Bartolini, A.; Tempesti, P.; Resta, C.; Berti, D.; Smets, J.; Baglioni, P.; Aouad, Y.G. Poly(ethylene glycol)-graft-poly(vinyl acetate) single-chain nanoparticles for the encapsulation of small molecules. Phys. Chem. Chem. Phys. 2017, 19, 4553–4559. [Google Scholar] [CrossRef]





| Sample | Optimal Active Component Concentration, % | ATPT Mass Difference, g | Sa, nm | k | ||||
|---|---|---|---|---|---|---|---|---|
| Scan Size, μm | ||||||||
| 1 | 5 | 10 | 1 | 5 | 10 | |||
| Paper | - | - | 51 | 272 | 789 | 1.06 | 1.26 | 1.53 |
| PS | 3 | 0.69 | - | |||||
| PEVA | 8 | 0.01 | 2 | 9 | 261 | 1.01 | 1.02 | 1.10 |
| PVOH | 8 | 0.12 | 0 | 142 | 531 | 1.00 | 1.03 | 1.12 |
| CC | 1 | 0.50 | 10 | 97 | 129 | 1.06 | 1.14 | 1.20 |
| Sample | Sa | k | Peel Test | Ink Drop Test | Link with Paper | Uniformity |
|---|---|---|---|---|---|---|
| PS | n/a | n/a | − | ± | − | − |
| PEVA | + | + | + | + | ± | + |
| PVOH | + | + | ± | − | − | + |
| CC | + | − | − | − | − | ± |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilev, S.; Vodyashkin, A.; Vasileva, D.; Zelenovskiy, P.; Chezganov, D.; Yuzhakov, V.; Shur, V.; O’Reilly, E.; Vinogradov, A. An Investigative Study on the Effect of Pre-Coating Polymer Solutions on the Fabrication of Low Cost Anti-Adhesive Release Paper. Nanomaterials 2020, 10, 1436. https://doi.org/10.3390/nano10081436
Vasilev S, Vodyashkin A, Vasileva D, Zelenovskiy P, Chezganov D, Yuzhakov V, Shur V, O’Reilly E, Vinogradov A. An Investigative Study on the Effect of Pre-Coating Polymer Solutions on the Fabrication of Low Cost Anti-Adhesive Release Paper. Nanomaterials. 2020; 10(8):1436. https://doi.org/10.3390/nano10081436
Chicago/Turabian StyleVasilev, Semen, Andrey Vodyashkin, Daria Vasileva, Pavel Zelenovskiy, Dmitry Chezganov, Vladimir Yuzhakov, Vladimir Shur, Emmet O’Reilly, and Alexandr Vinogradov. 2020. "An Investigative Study on the Effect of Pre-Coating Polymer Solutions on the Fabrication of Low Cost Anti-Adhesive Release Paper" Nanomaterials 10, no. 8: 1436. https://doi.org/10.3390/nano10081436