Thermal and Mechanical Characterization of an Aeronautical Graded Epoxy Resin Loaded with Hybrid Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SiO2@PDA Synthesis and Nanocomposites Manufacturing
2.3. SiO2@PDA Characterization
2.4. Nanocomposites Thermal and Mechanical Characterization
3. Results and Discussion
3.1. SiO2@PDA Characterization
3.2. Thermogravimetric Analysis: Experimental Results and Kissinger Model
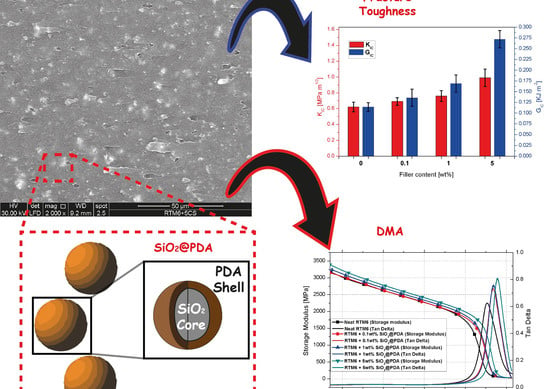
3.3. Nanocomposites Dynamic-Mechanical Properties
- Increase in glassy storage modulus: Different authors [31,32] have already reported this behavior in epoxy systems loaded with silica nanoparticles. The increase in storage modulus is attributable to the remarkable mismatch between the elastic modulus of epoxy matrix (3.19 GPa) and silica (70 GPa [33]);
- Increase in rubbery storage modulus: The reduction in crosslinking density in the hosting matrix can lower the elastic modulus in the rubbery region. Although the presence of the CSNPs actually limits the crosslinking mechanism of the epoxy polymeric chains, the CSNPs/RTM6 systems show an increase in the rubbery modulus with the increase in filler content. This behavior can be explained considering that the strong interfacial interactions, due to the PDA shell, allow the SiO2@PDA to act as a physical crosslinker. By a classical rubber elasticity equation [18] it is possible to estimate the apparent number of active network chain segments per unit volume (n):where E is the storage modulus at the onset of the rubbery plateau, R is the ideal gas constant and T is the absolute temperature corresponding to the E value. Figure 9a shows that n raises with the addition of CSNPs, demonstrating the ability of SiO2@PDA to act as a physical crosslinker, as shown in Figure 9b.E = 3nRT
- Increase in Tg: Different authors report that the addition of silica NPs can induce a reduction in Tg [32] or it could keep it unchanged [34]. It is well known that the increase in Tg in the case of a nanocomposite system likely depends by the ability of the filler to hinder the thermal motion of the polymeric hosting matrix chains. Thus, in the absence of bonds (physical or chemical) between hosting matrix and filler, the presence of the particles can induce a detrimental effect on the glass transition temperature of the neat matrix. Since SiO2@PDA acts as a physical crosslinker, the motion of the polymeric chains is efficiently hindered by their presence, with a consequent increase in the final glass transition temperature of the nanocomposite system;
- Increase in tan delta peak height: Tan delta is expressed as the ratio between the dissipative modulus (E’’) and the storage modulus (E’), and consequently the height of the tan delta peak is a measure of the material damping performance. Allahverdi et al. [35] have demonstrated that the addition of silica NPs will induce a reduction in the tan delta peak height, and this behavior is attributed to the inorganic nature of the silica particles which prevents energy dissipation. The presence of the PDA shell, instead, ensures an efficient dissipation due to its polymeric (and consequently viscoelastic behavior) nature, confirming the clear enhancement of damping performance for the SiO2@PDA/RTM6 nanocomposites.
3.4. Nanocomposites Fracture Toughness Performances
- Crack pinning is a typical fracture mechanism in polymeric systems loaded with inorganic particles. Hard fillers obstruct the propagation of the crack front, increasing the fracture toughness by bowing out the crack front between the NPs [36];
- The strong adhesion between filler and matrix promotes a severe triaxial stress state around the CSNPs, inducing a plastic deformation mechanism in the hosting matrix and causing crack tip blunting [37];
- The further plastic deformation of the matrix due to the presence of the PDA shell itself [38].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascault, J.P.; Sautereau, H.; Verdu, J.; Williams, R.J.J. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Hodgkin, J.H.; Simon, G.P.; Verley, R.J. Thermoplastic toughening of epoxy resins: A critical review. Polym. Adv. Tech. 1998, 9, 3–10. [Google Scholar] [CrossRef]
- Armstrong, K.B.; Graham, B.L.; Cole, W.F. Care and Repair of Advanced Composites, 2th ed.; Society of Automotive Engineers: Portland, OR, USA, 2005. [Google Scholar]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. The effect of glassy and rubbery hyperbranched polymers as modifiers in epoxy aeronautical systems. Compos. B Eng. 2019, 169, 88–95. [Google Scholar] [CrossRef]
- Liu, S.; Fan, X.; He, C. Improving the Fracture Toughness of Epoxy with Nanosilica-Rubber Core-Shell Nanoparticles. Compos. Sci. Technol. 2016, 125, 132–140. [Google Scholar] [CrossRef]
- Dillingham, R.G.; Boer, F.J. Interphase composition in aluminum/epoxy adhesive joints. J. Adhesion 1987, 24, 315–335. [Google Scholar] [CrossRef]
- Marotta, A.; Lama, G.C.; Ambrogi, V.; Cerruti, P.; Giamberini, M.; Gentile, G. Shape memory behavior of liquid-crystalline elastomer/graphene oxide nanocomposites. Compos. Sci. Technol. 2018, 159, 251–258. [Google Scholar] [CrossRef]
- Yee, A.F.; Pearson, R.A. Toughening mechanisms in elastomer-modified epoxies: Part 2. J. Mater. Sci. 1986, 21, 2475–2488. [Google Scholar] [CrossRef] [Green Version]
- Zotti, A.; Elmahdy, A.; Zuppolini, S.; Borriello, A.; Verleysen, P.; Zarrelli, M. Aromatic Hyperbranched Polyester/RTM6 Epoxy Resin for EXTREME Dynamic Loading Aeronautical Applications. Nanomaterials 2020, 10, 188. [Google Scholar] [CrossRef] [Green Version]
- Marouf, B.T.; Mai, Y.W.; Bagheri, R.; Pearson, R.A. Toughening of Epoxy Nanocomposites: Nano and Hybrid Effects. Polym. Rev. 2016, 56, 70–112. [Google Scholar] [CrossRef]
- Kim, J.A.; Seong, D.G.; Kang, T.J.; Youn, J.R. Effects of surface modification on rheological and mechanical properties of CNT/epoxy composites. Carbon 2006, 4, 1898–1905. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, P.; Li, G.; Lu, D.; Sun, R.; Wong, C. Core-shell SiO2@RGO hybrids for epoxy composites with low percolation threshold and enhanced thermo-mechanical properties. J. Mater. Chem. A 2014, 2, 18246–18255. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal Properties and Fracture Toughness of Epoxy Nanocomposites Loaded with Hyperbranched-Polymers-Based Core/Shell Nanoparticles. Nanomaterials 2019, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical propertiesof particulate–polymer composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Sohn, J.E. Improved Matrix-Filler Adhesion. J. Adhesion. 1985, 19, 15–27. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface Modification of Aramid Fibers by Bio-Inspired Poly(dopamine) and Epoxy Functionalized Silane Grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Phua, S.L.; Teo, J.K.H.; Toh, C.L.; Lau, S.K.; Ma, J.; Lu, X. A Biomimetic Approach to Enhancing Interfacial Interactions: Polydopamine-Coated Clay as Reinforcement for Epoxy Resin. ACS Appl. Mater. Interfaces 2011, 3, 3026–3032. [Google Scholar] [CrossRef]
- Subramanian, A.S.; Tey, J.N.; Zhang, L.; Ng, B.H.; Roy, S.; Wei, J.; Hu, X. Synergistic bond strengthening in epoxy adhesives using polydopamine/MWCNT hybrids. Polymer 2016, 82, 285–294. [Google Scholar] [CrossRef]
- Morelle, X.P.; Lani, F.; Melchior, M.A.; André, S.; Bailly, C.; Pardoen, T. The Elasto-Viscoplasticity and Fracture Behaviour of the RTM6 Structural Epoxy and Impact on the Response of Woven Composites. In Proceedings of the 15th European Conference on Composite Materials (ECCM15), Venice, Italy, 24–28 June 2012. [Google Scholar]
- Technical Data Bulletin RTM6; Hexcel: Stanford, CT, USA, 2016.
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Effect of SiO2@polydopamine core/shell nanoparticles as multifunctional filler for an aeronautical epoxy resin. Mater. Today Proc. 2020. (In press) [Google Scholar] [CrossRef]
- Kalali, E.N.; Wang, X.; Wang, D.Y. Multifunctional intercalation in layered double hydroxide: Toward multifunctional nanohybrid for epoxy resin. J. Mater. Chem. A 2016, 4, 2147–2157. [Google Scholar] [CrossRef] [Green Version]
- Doyle, C.D. Estimating Thermal Stability of Experimental Polymers by Empirical Thermogravimetric Analysis. Anal. Chem. 1961, 33, 77–79. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, D.I.; Lee, J.R. Surface Modification of Montmorillonite on Surface Acid–Base Characteristics of Clay and Thermal Stability of Epoxy/Clay Nanocomposites. J. Colloid Interface Sci. 2002, 251, 160–165. [Google Scholar] [CrossRef]
- Wellen, R.M.R.; Canedo, E.L. On the Kissinger equation and the estimate of activation energies for non-isothermal cold crystallization of PET. Polym. Test. 2014, 40, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and Kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.K.; Kim, S.H. Thermal Decomposition Behavior of Poly(ethylene 2,6-naphthalate)/Silica Nanocomposites. Polym. Compos. 2009, 30, 1779–1787. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Yu, J.; Chen, L.; Zhu, J.; Hu, Z. Polydopamine particles for next-generation multifunctional biocomposites. J. Mater. Chem. A 2014, 2, 7578–7587. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Cho, J.H.; Iyer, P.; Baranowitz, S.; Ellison, C.J. Thermooxidative Stabilization of Polymers Using Natural and Synthetic Melanins. Macromolecules 2011, 44, 9499–9507. [Google Scholar] [CrossRef]
- Hsieh, T.H.A.J.; Kinloch, K.; Masania, J.; Sohn Lee, A.C.; Taylor, S.; Sprenger, S. The toughness of epoxy polymers and fibre composites modifiedwith rubber microparticles and silica nanoparticles. J. Mater. Sci. 2010, 45, 1193–1210. [Google Scholar] [CrossRef]
- Ragosta, G.; Abbate, M.; Musto, P.; Scarinzi, G.; Masci, L. Epoxy-silica particulate nanocomposites: Chemical interactions, reinforcement and fracture toughness. Polymer 2005, 46, 10506–10516. [Google Scholar] [CrossRef]
- Pascoe, K.J. An Introduction to the Properties of Engineering Materials, 3rd ed.; Van, N.R., Ed.; Springer Science & Business Media: London, UK, 1978. [Google Scholar]
- Johnsen, B.B.; Kinloch, A.J.; Mohammed, R.D.; Taylor, A.C.; Sprenger, S. Toughening mechanisms of nanoparticle-modified epoxy polymers. Polymer 2007, 48, 530–541. [Google Scholar] [CrossRef]
- Allahverdi, A.; Ehsani, M.; Janpour, H.; Ahmadi, S. The effect of nanosilica on mechanical, thermal and morphological properties of epoxy coating. Prog. Org. 2012, 75, 543–548. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Fracture Toughening Mechanisms in Epoxy Adhesives; InTech: London, UK, 2016; pp. 237–269. [Google Scholar]
- Kinloch, A.J.; Maxwell, D.; Young, R.J. Micromechanisms of crack propagation in hybrid particulate composites. J. Mater. Sci. Lett. 1985, 4, 1276–1279. [Google Scholar] [CrossRef]
- Wanshuang, L.; Wang, Y.; Wang, P.; Li, Y.; Jiang, Q.; Hu, X.; Wei, Y.; Qiu, Y.; Shahabadi, S.I.S.; Lu, X. A biomimetic approach to improve the dispersibility, interfacial interactions and toughening effects of carbon nanofibers in epoxy composites. Compos. B Eng. 2017, 113, 197–205. [Google Scholar]











| Sample | T10 (°C) | T80 (°C) | TMAX (°C) | IPDT (°C) | Weight Residual (@ 600 °C) (%) |
|---|---|---|---|---|---|
| Neat RTM6 | 360.5 ± 1.3 | 444.4 ± 1.0 | 391.8 ± 0.1 | 479.7 | 9.05 ± 0.3 |
| RTM6 + 0.1 wt%SiO2@PDA | 363.4 ± 1.2 | 454.4 ± 5.5 | 394.5 ± 0.9 | 482.7 | 9.09 ± 0.2 |
| RTM6 + 1 wt%SiO2@PDA | 365.0 ± 0.9 | 455.9 ± 0.8 | 394.6 ± 0.9 | 492.8 | 10.19 ± 0.5 |
| RTM6 + 5 wt%SiO2@PDA | 370.5 ± 0.5 | 464.7 ± 2.6 | 395.9 ± 1.1 | 510.5 | 13.03 ± 0.3 |
| Sample | Storage Modulus (at 30 °C) (MPa) | Storage Modulus (at 260 °C) (MPa) | Tg (°C) |
|---|---|---|---|
| Neat RTM6 | 3192 ± 21.0 | 25.1 ± 1.5 | 228.9 ± 0.5 |
| RTM6 + 0.1 wt% SiO2@PDA | 3200 ± 16.0 | 29.5 ± 1.5 | 233.6 ± 0.2 |
| RTM6 + 1 wt% SiO2@PDA | 3275 ± 19.0 | 30.5 ± 1.0 | 234.3 ± 0.4 |
| RTM6 + 5 wt% SiO2@PDA | 3434 ± 8.0 | 33.1 ± 0.7 | 239.0 ± 0.2 |
| Sample | KIC (MPa·m1/2) | % Variation KIC | GIC (KJ·m2) | % Variation GIC |
|---|---|---|---|---|
| Neat RTM6 | 0.62 ± 0.06 | - | 0.114 ± 0.01 | - |
| RTM6 + 0.1 wt%SiO2@PDA | 0.69 ± 0.05 | 11.3 | 0.136 ± 0.02 | 19.3 |
| RTM6 + 1 wt%SiO2@PDA | 0.76 ± 0.07 | 22.6 | 0.169 ± 0.02 | 48.3 |
| RTM6 + 5 wt%SiO2@PDA | 0.99 ± 0.11 | 60.0 | 0.272 ± 0.02 | 138.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Thermal and Mechanical Characterization of an Aeronautical Graded Epoxy Resin Loaded with Hybrid Nanoparticles. Nanomaterials 2020, 10, 1388. https://doi.org/10.3390/nano10071388
Zotti A, Zuppolini S, Borriello A, Zarrelli M. Thermal and Mechanical Characterization of an Aeronautical Graded Epoxy Resin Loaded with Hybrid Nanoparticles. Nanomaterials. 2020; 10(7):1388. https://doi.org/10.3390/nano10071388
Chicago/Turabian StyleZotti, Aldobenedetto, Simona Zuppolini, Anna Borriello, and Mauro Zarrelli. 2020. "Thermal and Mechanical Characterization of an Aeronautical Graded Epoxy Resin Loaded with Hybrid Nanoparticles" Nanomaterials 10, no. 7: 1388. https://doi.org/10.3390/nano10071388