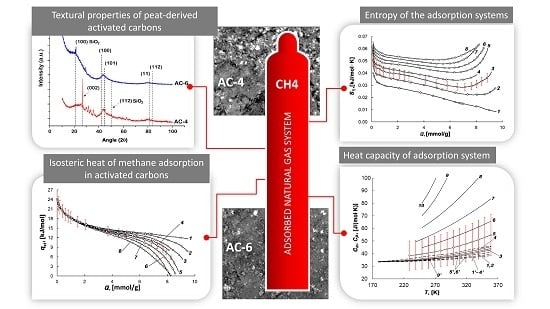
Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbent
2.2. Adsorptive
2.3. Methods
3. Results and Discussion
3.1. Structure and Morphology Characterization
3.2. Methane Adsorption on the Peat-Derived Carbon Adsorbents
3.3. Calculation of Thermodynamic Functions of Adsorption Systems
3.3.1. Differential Molar Isosteric Heat of Adsorption
3.3.2. Integral Heat of Methane Adsorption in the Activated Carbons
3.3.3. Differential Molar Isosteric Entropy of the Methane-AC Adsorption Systems
3.3.4. Differential Molar Isosteric Enthalpy of the Methane-AC Adsorption Systems
3.3.5. Differential Molar Isosteric Heat Capacity of the Methane-AC Adsorption Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nie, Z.; Lin, Y.; Jin, X. Research on the theory and application of adsorbed natural gas used in new energy vehicles: A review. Front. Mech. Eng. 2016, 11, 258–274. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Porous carbon-based adsorption systems for natural gas (methane) storage. Russ. Chem. Rev. 2018, 87, 950–983. [Google Scholar] [CrossRef]
- Kumar, K.V.; Preuss, K.; Titirici, M.M.; Rodríguez-Reinoso, F. Nanoporous materials for the onboard storage of natural gas. Chem. Rev. 2017, 117, 1796–1825. [Google Scholar] [CrossRef]
- Men’shchikov, I.E.; Fomkin, A.A.; Tsivadze, A.Y.; Shkolin, A.V.; Strizhenov, E.M.; Khozina, E.V. Adsorption accumulation of natural gas based on microporous carbon adsorbents of different origin. Adsorption J. 2017, 23, 327–339. [Google Scholar] [CrossRef]
- Elizabeth Casco, M.; Martínez-Escandell, M.; Gadea-Ramos, E. High-pressure methane storage in porous materials: Are carbon materials in the pole position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Fomkin, A.A.; Men’shchikov, I.E.; Pribylov, A.A.; Shkolin, A.V.; Zaitsev, D.S.; Tvardovski, A.V. Methane adsorption in microporous carbon adsorbent with wide distribution of pores. Colloid J. 2017, 79, 144–151. [Google Scholar] [CrossRef]
- Shevchenko, A.O.; Pribylov, A.A.; Zhedulov, S.A.; Men’shchikov, I.E.; Shkolin, A.V.; Fomkin, A.A. Methane adsorption in microporous carbon adsorbent LCN obtained by thermochemical synthesis from lignocellulose. Prot. Met. Phys. Chem. Surf. 2019, 55, 211–216. [Google Scholar] [CrossRef]
- Policicchio, A.; Maccallini, E.; Agostino, R.G.; Ciuchi, F.; Aloise, A.; Giordano, G. Higher methane storage at low pressure and room temperature in new easily scalable large-scale production activated carbon for static and vehicular applications. Fuel 2013, 104, 813–821. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.V.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Makal, T.A.; Li, J.-R.; Lu, W.; Zhou, H.-C. Methane storage in advanced porous materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.-C. Highly stable porous polymer networks with exceptionally high gas-uptake capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal-organic frameworks. Polyhedron 2018, 145, 1–15. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Chupas, P.J. Pressure-induced amorphization, and porosity modification in a metal-organic framework. J. Am. Chem. 2009, 131, 17546–17547. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Rizzo, C.; Millini, R.; Dietzel, P.D.C.; Blom, R.; Zanardi, S. Methane storage on CPO-27-Ni pellets. J. Porous Mater. 2011, 18, 289–296. [Google Scholar] [CrossRef]
- Zhang, H.; Deria, P.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. A thermodynamic tank model for studying the effect of higher hydrocarbons on natural gas storage in metal-organic frameworks. Energy Environ. Sci. 2015, 8, 1501–1510. [Google Scholar] [CrossRef]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic analysis of metal−organic frameworks for hydrogen and natural gas storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. Activated carbon and adsorption. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 22–34. [Google Scholar]
- Uraki, Y.; Tamai, Y.; Ogawa, M.; Gaman, S.; Tokurad, S. Preparation of activated carbon from peat. BioResources 2009, 4, 205–213. [Google Scholar]
- Mukhin, V.M.; Tarasov, A.V.; Klushin, V.N. Aktivnie Ugli Rossii (Active Carbons of Russia); Metallurgiya: Moscow, Russia, 2000; p. 352. [Google Scholar]
- Dubinin, M.M. Physical adsorption of gases and vapors in micropores. Prog. Surf. Membr. Sci. 1975, 9, 1–70. [Google Scholar]
- WanDaud, W.M.A.; Houshamnd, A.H. Textural characteristics, surface chemistry, and oxidation of activated carbon. J. Nat. Gas. Chem. 2010, 19, 267–279. [Google Scholar]
- Myers, A.L. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Wang, Y.; Ercan, C.; Khawajah, A.; Othman, R. Experimental and theoretical study of methane adsorption on granular activated carbons. AIChE J. 2012, 58, 782–788. [Google Scholar] [CrossRef]
- Rahman, K.A.; Loh, W.S.; Ng, K.C. Heat of adsorption and adsorbed phase specific heat capacity of methane/activated carbon system. Procedia Eng. 2013, 56, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Bakaev, V.A. Molecular Theory of Physical Adsorption. Ph.D. Thesis, Moscow State University, Moscow, Russia, May 1990. (In Russian). [Google Scholar]
- Bakaev, V.A. One possible formulation of the thermodynamics of sorption equilibrium. Bull. Acad. Sci. USSR Div. Chem. Sci. 1971, 20, 2516–2520. [Google Scholar] [CrossRef]
- Guggenheim, E.A. Modern Thermodynamics by the Methods of Willard Gibbs; Methuen & Co. Ltd.: London, UK, 1933; p. 206. [Google Scholar]
- Guggenheim, E.A. Thermodynamics. An Advanced Treatment for Chemist and Physicist; North Holland Publishing Company: Amsterdam, The Netherlands, 1967; pp. 166–169. [Google Scholar]
- Hill, T.L. Theory of Physical Adsorption in Advances in Catalysis and Related Subjects; Academic Press: New York, NY, USA, 1952; Volume 4, pp. 211–258. [Google Scholar]
- Gibbs, J.V. Thermodynamic Works; Gostekhizdat: Moscow, Russia, 1950; p. 492. (In Russian) [Google Scholar]
- Fomkin, A.A. Adsorption of gases, vapors and liquids by microporous adsorbents. Adsorption 2005, 11, 425–436. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A. Thermodynamics of methane adsorption on the microporous carbon adsorbent ACC. Russ. Chem. Bull. 2008, 57, 1799–1805. [Google Scholar] [CrossRef]
- Strizhenov, E.M.; Shkolin, A.V.; Fomkin, A.A.; Sinitsyn, V.A.; Zherdev, A.A.; Smirnov, I.A.; Pulin, A.L. Low-temperature adsorption of methane on microporous AU-1 carbon adsorbent. Prot. Met. Phys. Chem. Surf. 2014, 50, 15–21. [Google Scholar]
- Jinmung, H.; Xuehui, M. Physical and chemical properties of peat. In Coal, Oil Shale, Natural Bitumen, Heavy Oil and Peat; EOLSS Publisher Co Ltd.: Oxford, UK, 2009; Volume II, pp. 315–318. [Google Scholar]
- Fenelonov, V.B. Poristyi Uglerod (Porous Carbon); Boreskov Institute of Catalysis SB RAS: Novosibirsk, Russia, 1995; p. 518. (In Russian) [Google Scholar]
- Manocha, S.M. Porous carbons. In Frontiers in Material Science; Universities Press Private Ltd.: Hyderabad, India, 2005; pp. 335–348. [Google Scholar]
- Rodriguez-Reinoso, F.; Molina-Sabio, M.; Gonzalez, M.T. The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 1995, 33, 15–23. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; González, M.T.; Rodriguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of steam and carbon dioxide activation in the micropore size distribution of activated carbon. Carbon 1996, 34, 505–509. [Google Scholar] [CrossRef]
- Sychev, V.V.; Vasserman, A.A.; Zagoruchenko, V.A.; Kozlov, A.D.; Spiridonov, G.A.; Tzymarnyi, V.A. Thermodynamic Properties of Methane; Izd. Standartov: Moscow, Russia, 1979; p. 349. (In Russian) [Google Scholar]
- Shkolin, A.V.; Fomkin, A.A. Measurement of carbon-nanotube adsorption of energy-carrier gases for alternative energy systems. Meas. Techn. 2018, 61, 395–401. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Men’shchikov, I.E.; Kharitonov, V.M.; Pulin, A.L. A bench for measuring the adsorption of gases and vapors by gravimetric technique and the method of its operational use. RF Patent 2019143065, 23 December 2019. [Google Scholar]
- GOST 34100.3-2017/ISO/IEC Guide 98-3:2008. Part 3. Uncertainty of measurement. Part 3. Guide to the expression of uncertainty in measurement. Available online: https://files.stroyinf.ru/Data/651/65118.pdf (accessed on 18 June 2020).
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Isirikyan, A.A.; Kiselev, A.V. The absolute adsorption isotherms of vapors of nitrogen, benzene, and n-hexane, and the heats of adsorption of benzene and n-hexane on graphitized carbon black. J. Phys. Chem. 1961, 65, 601–607. [Google Scholar] [CrossRef]
- Kiselev, A.V. New adsorption method for the determination of surface of adsorbent. Uspekhi Khimii 1945, XVI, 367–394. (In Russian) [Google Scholar]
- Shkolin, A.V.; Fomkin, A.A.; Pulin, A.L.; Yakovlev, V.Y. A technique for measuring an adsorption-induced deformation. Instrum. Exp. Tech. 2008, 51, 150–155. [Google Scholar] [CrossRef]
- Fomkin, A.A.; Shkolin, A.V.; Men’shchikov, I.E.; Pulin, A.L.; Pribylov, A.A.; Smirnov, I.A. Measurement of adsorption of methane at high pressures for alternative energy systems. J. Meas. Techn. 2016, 58, 1387–1391. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Serpinskii, V.V.; Kalashnikov, S.M. Adsorption of gases by microporous adsorbents under pressures up to hundreds of megapascals. Zeolites 1991, 11, 846–849. [Google Scholar] [CrossRef]
- Downarowicz, D. Adsorption characteristics of propane-2-ol vapours on activated carbon Sorbonorit 4 in electrothermal temperature swing adsorption process. Adsorption 2015, 21, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Bergna, D.; Hu, T.; Prokkola, H.; Romar, H.; Lassi, U. Effect of some process parameters on the main properties of activated carbon produced from peat in a lab-scale process. Waste Biomass Valorization 2020, 11, 2837–2848. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, L.A.; Svergun, D.I. Structure Analysis by Small-Angle X-Ray and Neutron Scattering; Plenum Press: New York, NY, USA, 1989; p. 335. [Google Scholar]
- Shiryaev, A.A.; Voloshchuk, A.M.; Volkov, V.V.; Averin, A.A.; Artamonova, S.D. Nanoporous active carbons at ambient conditions: A comparative study using X-ray scattering and diffraction, Raman spectroscopy and N2 adsorption. J. Phys. Conf. Series 2017, 848, 012009. [Google Scholar] [CrossRef]
- Guinier, A. La diffraction des rayons X aux tres petits angles: Application a l’etude de phenomenes ultramicroscopiques. Ann. Phys. 1939, 11, 161–237. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Plavnik, G.M. Microporous structures of carbonaceous adsorbents. Carbon 1968, 6, 183–192. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Erashko, I.T.; Kadlec, O.; Ulin, V.I.; Voloshchuk, A.M.; Zolotarev, P.P. Kinetics of physical adsorption by carbonaceous adsorbents of biporous structure. Carbon 1975, 13, 193–200. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Stoeckli, H.F. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Bakaev, V.A. The statistical thermodynamics of adsorption equilibriums in the case of zeolites. Dokl. Acad. Nauk SSSR 1966, 167, 369–372. [Google Scholar]
- Pribylov, A.A.; Murdmaa, K.A. Adsorption of gases onto Polymer Adsorbent MN-270 in the region of supercritical temperatures and pressures. Prot. Met. Phys. Chem. Surf. 2020, 56, 115–121. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Kalinnikova, I.A.; Murdmaa, K.O. Determination of the average heat and characteristic energy from the adsorption isotherm. Russ. Chem. Bull. Int. Ed. 2016, 65, 972–977. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Fomkin, A.A.; Tsivadze, A.Y.; Anuchin, K.M.; Men’shchikov, I.E.; Pulin, A.L. Experimental study and numerical modeling: Methane adsorption in microporous carbon adsorbent over the subcritical and supercritical temperature regions. Prot. Met. Phys. Chem. Phys. Surf. 2016, 52, 955–963. [Google Scholar] [CrossRef]
- Novikova, S.I. Teplovoe Rasshirenie Tverdykh Tel (Heat Expansion of Solids); Nauka: Moscow, Russia, 1974; p. 293. (In Russian) [Google Scholar]
- Yeganegi, S.; Gholampour, F. Methane adsorption and diffusion in a model nanoporous carbon: An atomistic simulation study. Adsorption 2013, 19, 979–987. [Google Scholar] [CrossRef]
- Nicholson, D.; Stubos, T. Simulation of adsorption in micropores. In Recent Advances in Gas Separation by Microporous Ceramic Membranes; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; Volume 6, pp. 231–256. [Google Scholar]
- Anuchin, K.M.; Fomkin, A.A.; Korotych, A.P.; Tolmachev, A.M. Adsorption concentration of methane. dependence of adsorbate density on the width of slit-shaped micropores in activated carbons. Prot. Met. Phys. Chem. Surf. 2014, 50, 173–177. [Google Scholar] [CrossRef]
- Kel’tsev, N.V. Osnovy Adsorbtsionnoi Praktiki (Fundamentals of Adsorption Practice); Khimiya: Moscow, Russia, 1976; p. 90. (In Russian) [Google Scholar]
- Torres-Knoop, A.; Poursaeidesfahani, A.; Vlugt, T.J.H.; Dubbeldam, D. Behavior of the enthalpy of adsorption in nanoporous materials close to saturation conditions. J. Chem. Theor. Comput. 2017, 13, 3326–3339. [Google Scholar] [CrossRef] [PubMed]
- Fomkin, A.A.; Serpinskii, V.V.; Bering, B.P. Investigation of the adsorption of xenon on NaX zeolite within a broad range of pressures and temperatures. Russ. Chem. Bull. 1975, 24, 1147–1150. [Google Scholar] [CrossRef]
- Chkhaidze, E.V.; Fomkin, A.A.; Serpinskii, V.V.; Tsitsishvili, G.V.; Dubinin, M.M. Methane adsorption on a microporous carbon adsorbent in the precritical and hypercritical regions. Russ. Chem. Bull. 1986, 35, 847–849. [Google Scholar] [CrossRef]
- Chkhaidze, E.V.; Fomkin, A.A.; Serpinskii, V.V.; Tsitsishvili, G.V. Thermodynamics of the adsorption of methane on NaX zeolite. Russ. Chem. Bull. 1986, 35, 252–255. [Google Scholar] [CrossRef]










| Sample | E0, kJ/mol | W0, cm3/g | x0, nm | SBET, m2/g | Wt, cm3/g | Smeso, m2/g | Wmeso, cm3/g |
|---|---|---|---|---|---|---|---|
| AC-4 | 20.6 | 0.48 | 0.58 | 957 | 0.72 | 105 | 0.24 |
| AC-6 | 19.1 | 0.60 | 0.63 | 1334 | 0.70 | 96 | 0.10 |
| Sample | Elements (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | K | S | Si | Al | Cl | Ca | Fe | Mg | B | |
| AC-4 | 74.9 | 10.7 | 0.8 | 3.0 | 2.4 | 0.6 | 0.6 | 1.4 | 0.3 | 0.18 | 4.9 |
| AC-6 | 60.8 | 23.7 | 0.5 | 1.7 | 10.2 | 1.2 | 0.6 | 1.3 | - | - | - |
| AC | T, K | 178.00 | 216.00 | 243.00 | 273.15 | 300.00 | 320.00 | 340.00 | 360.00 |
|---|---|---|---|---|---|---|---|---|---|
| AC-4 | P, MPa | 1.0 | 3.4 | 6.2 | 5.1 | 13.0 | 16.0 | 18.0 | 23.0 |
| a, mmol/g | 9.6 | 9.6 | 9.2 | 8.1 | 9.0 | 8.7 | 8.3 | 8.3 | |
| Q, kJ/kg | 141.4 | 135.8 | 126.6 | 115.9 | 116.9 | 110.4 | 104.1 | 99.3 | |
| ∆T, K | 58.8 | 51.9 | 45.2 | 46.0 | 39.4 | 37.0 | 35.2 | 32.6 | |
| AC-6 | P, MPa | 1.0 | 4.0 | 5.7 | 5.5 | 15.0 | 19.0 | 21.0 | 23.0 |
| a, mmol/g | 11.9 | 11.9 | 11.0 | 10.0 | 11.1 | 10.9 | 10.4 | 10.1 | |
| Q, kJ/kg | 182.1 | 174.1 | 159.4 | 147.7 | 149.3 | 140.9 | 133.8 | 128.1 | |
| ∆T, K | 69.3 | 58.6 | 53.8 | 53.6 | 44.4 | 41.4 | 40.0 | 38.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Men’shchikov, I.; Shkolin, A.; Khozina, E.; Fomkin, A. Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons. Nanomaterials 2020, 10, 1379. https://doi.org/10.3390/nano10071379
Men’shchikov I, Shkolin A, Khozina E, Fomkin A. Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons. Nanomaterials. 2020; 10(7):1379. https://doi.org/10.3390/nano10071379
Chicago/Turabian StyleMen’shchikov, Ilya, Andrey Shkolin, Elena Khozina, and Anatoly Fomkin. 2020. "Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons" Nanomaterials 10, no. 7: 1379. https://doi.org/10.3390/nano10071379