Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
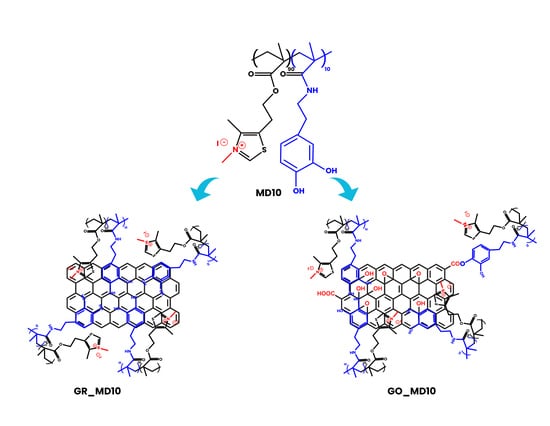
2.2. Synthesis of GO and GR
2.3. GO and GR Functionalization
2.4. Characterization of Hybrid Materials
2.5. Antimicrobial Activity
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The antibacterial applications of graphene and its derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Zhang, B.; Tang, J. Mussel-inspired functionalization of graphene for synthesizing ag-polydopamine-graphenenanosheets as antibacterial materials. Nanoscale 2013, 5, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Tria, M.C.; Vergara, R.A.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial graphene polymer (pvk-go) nanocomposite films. Chem. Commun. (Camb.) 2011, 47, 8892–8894. [Google Scholar] [CrossRef]
- Santos, C.M.; Milagros Cui, K.; Ahmed, F.; Tria, M.C.R.; Vergara, R.A.M.V.; de Leon, A.C.; Advincula, R.C.; Rodrigues, D.F. Bactericidal and anticorrosion properties in pvk/mwnt nanocomposite coatings on stainless steel. Macromol. Mater. Eng. 2012, 297, 807–813. [Google Scholar] [CrossRef]
- Pangilinan, K.D.; Santos, C.M.; Estillore, N.C.; Rodrigues, D.F.; Advincula, R.C. Temperature-responsiveness and antimicrobial properties of cnt-pnipam hybrid brush films. Macromol. Chem. Phys. 2013, 214, 464–469. [Google Scholar] [CrossRef]
- Aslan, S.; Deneufchatel, M.; Hashmi, S.; Li, N.; Pfefferle, L.D.; Elimelech, M.; Pauthe, E.; Van Tassel, P.R. Carbon nanotube-based antimicrobial biomaterials formed via layer-by-layer assembly with polypeptides. J. Colloid Interface Sci. 2012, 388, 268–273. [Google Scholar] [CrossRef]
- Mejias Carpio, I.E.; Santos, C.M.; Wei, X.; Rodrigues, D.F. Toxicity of a polymer-graphene oxide composite against bacterial planktonic cells, biofilms, and mammalian cells. Nanoscale 2012, 4, 4746–4756. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Placha, D.; Jampilek, J. Graphenic materials for biomedical applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; Al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Sangsri, T.; Siwayaprahm, P. Synthesis and antifungal activity of reduced graphene oxide nanosheets. Carbon 2012, 50, 5156–5161. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and antibiofilm efficacy of graphene oxide against chronic wound microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.-C.; Lee, P.-L.; Chou, Y.-C.; Wang, Y.-S. Antibacterial property of graphene oxide: The role of phototransformation. Environ. Sci. Nano 2017, 4, 647–657. [Google Scholar] [CrossRef]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene oxide mediated broad-spectrum antibacterial based on bimodal action of photodynamic and photothermal effects. Front. Microbiol. 2019, 10, 2995. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.N.; Huang, N.M.; Loo, C.H. Facile preparation of graphene-based chitosan films: Enhanced thermal, mechanical and antibacterial properties. J. Non Cryst. Solids 2012, 358, 525–530. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Wang, H.; Zhang, X.; Xu, W.; Li, Y.; Li, Q.; Wei, G.; Su, Z. Graphene film doped with silver nanoparticles: Self-assembly formation, structural characterizations, antibacterial ability, and biocompatibility. Biomater. Sci. 2015, 3, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Shi, X.; Cheng, L.; Luo, Y.; Dong, Z.; Gong, H.; Xu, L.; Zhong, Z.; Peng, R.; Liu, Z. Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS Appl. Mater. Interfaces 2014, 6, 8542–8548. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; De-La-Pinta, I.; Quindos, G.; Fernandez, M.J.; Fernandez, M.D. Graphene oxide-silver nanoparticle nanohybrids: Synthesis, characterization, and antimicrobial properties. Nanomaterials 2020, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Kong, W.; Sun, M.; Yang, Y.; Liu, W.; Lv, M.; Song, S.; Wang, L.; Song, H.; Hao, R. Highly stable graphene-based nanocomposite (GO-PEI-Ag) with broad-spectrum, long-term antimicrobial activity and antibiofilm effects. ACS Appl. Mater. Interfaces 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Raja, A.; Selvakumar, K.; Rajasekaran, P.; Arunpandian, M.; Ashokkumar, S.; Kaviyarasu, K.; Asath Bahadur, S.; Swaminathan, M. Visible active reduced graphene oxide loaded titania for photodecomposition of ciprofloxacin and its antibacterial activity. Colloids Surf. Physicochem. Eng. Asp. 2019, 564, 23–30. [Google Scholar] [CrossRef]
- Hsueh, Y.H.; Hsieh, C.T.; Chiu, S.T.; Tsai, P.H.; Liu, C.Y.; Ke, W.J. Antibacterial property of composites of reduced graphene oxide with nano-silver and zinc oxide nanoparticles synthesized using a microwave-assisted approach. Int. J. Mol. Sci. 2019, 20, 5394. [Google Scholar] [CrossRef] [Green Version]
- Archana, S.; Kumar, K.Y.; Jayanna, B.K.; Olivera, S.; Anand, A.; Prashanth, M.K.; Muralidhara, H.B. Versatile graphene oxide decorated by star shaped zinc oxide nanocomposites with superior adsorption capacity and antimicrobial activity. J. Sci. Adv. Mater. Devices 2018, 3, 167–174. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nadres, E.T.; Alamani, B.G.; Rodrigues, D.F. Designing polymeric adhesives for antimicrobial materials: Poly(ethylene imine) polymer, graphene, graphene oxide and molybdenum trioxide - a biomimetic approach. J. Mater. Chem. B 2017, 5, 6616–6628. [Google Scholar] [CrossRef]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide–chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Liu, M.; Wang, Y.; He, W.; Shi, G.; Hu, X.; Zhan, R.; Luo, G.; Xing, M.; et al. Synthesis of graphene oxide-quaternary ammonium nanocomposite with synergistic antibacterial activity to promote infected wound healing. Burn. Trauma 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Tian, C.; Ma, T.; Pang, L.; Wang, J. Click synthesis of quaternized poly(dimethylaminoethyl methacrylate) functionalized graphene oxide with improved antibacterial and antifouling ability. Colloids Surf. B Biointerfaces 2016, 141, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Chiloeches, A.; Echeverría, C.; Cuervo-Rodríguez, R.; Plachà, D.; López-Fabal, F.; Fernández-García, M.; Muñoz-Bonilla, A. Adhesive antibacterial coatings based on copolymers bearing thiazolium cationic groups and catechol moieties as robust anchors. Prog. Org. Coat. 2019, 136, 105272. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Guadagno, L.; Raimondo, M.; Vertuccio, L.; Mauro, M.; Guerra, G.; Lafdi, K.; De Vivo, B.; Lamberti, P.; Spinelli, G.; Tucci, V. Optimization of graphene-based materials outperforming host epoxy matrices. RSC Adv. 2015, 5, 36969–36978. [Google Scholar] [CrossRef] [Green Version]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905. [Google Scholar] [CrossRef]
- Kim, S.-G.; Park, O.-K.; Lee, J.H.; Ku, B.-C. Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett. 2013, 14, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Vidano, R.P.; Fischbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T.L. Probing layer number and stacking order of few-layer graphene by raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Chiloeches, A.; Echeverría, C.; Fernández-García, M.; Muñoz-Bonilla, A. Influence of polymer composition and substrate on the performance of bioinspired coatings with antibacterial activity. Coatings 2019, 9, 733. [Google Scholar] [CrossRef] [Green Version]
- Gurzęda, B.; Florczak, P.; Wiesner, M.; Kempiński, M.; Jurga, S.; Krawczyk, P. Graphene material prepared by thermal reduction of the electrochemically synthesized graphite oxide. RSC Adv. 2016, 6, 63058–63063. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.R.; Phillips, D.J.; Wilson, N.R.; Gibson, M.I.; Rourke, J.P. One-step grafting of polymers to graphene oxide. Polym. Chem. 2015, 6, 8270–8274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larciprete, R.; Fabris, S.; Sun, T.; Lacovig, P.; Baraldi, A.; Lizzit, S. Dual path mechanism in the thermal reduction of graphene oxide. J. Am. Chem. Soc. 2011, 133, 17315–17321. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Falentin-Daudré, C.; Jérôme, C.; Lyskawa, J.; Fournier, D.; Woisel, P.; Detrembleur, C. Catechols as versatile platforms in polymer chemistry. Prog. Polym. Sci. 2013, 38, 236–270. [Google Scholar] [CrossRef]
- Kaminska, I.; Das, M.R.; Coffinier, Y.; Niedziolka-Jonsson, J.; Sobczak, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Boukherroub, R.; Szunerits, S. Reduction and functionalization of graphene oxide sheets using biomimetic dopamine derivatives in one step. ACS Appl. Mater. Interfaces 2012, 4, 1016–1020. [Google Scholar] [CrossRef]
- Vallés, C.; Drummond, C.; Saadaoui, H.; Furtado, C.A.; He, M.; Roubeau, O.; Ortolani, L.; Monthioux, M.; Pénicaud, A. Solutions of negatively charged graphene sheets and ribbons. J. Am. Chem. Soc. 2008, 130, 15802–15804. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, L.; Jiang, X.; Liu, R.; Wei, Y.; Wang, Y.; Zhao, Y.; Chai, Z.; Gao, X. Positively charged graphene oxide nanoparticle: Precisely label the plasma membrane of live cell and sensitively monitor extracellular ph in situ. Chem. Commun. (Camb.) 2014, 50, 3695–3698. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Liang, S.; Liu, L.; Zhang, X.; Ma, S. Water can stably disperse liquid-exfoliated graphene. Chem. Commun. (Camb.) 2013, 49, 11059–11061. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6, 3449–3459. [Google Scholar] [CrossRef] [Green Version]








| Material | Td5 (°C) | Tdmax1 (°C) | Tdmax2 (°C) | Tdmax3 (°C) | Tdmax4 (°C) |
|---|---|---|---|---|---|
| MD10 | 202 | 221 | 316 | 511 | - |
| GO | 158 | 235 | 483 | - | - |
| GO_MD10 | 185 | 188 | 320 | 533 | - |
| GR | 628 | 760 | - | - | - |
| GR_MD10 | 296 | 210 | 347 | 484 | 762 |
| Material | ζ Potential (mV) |
|---|---|
| MD10 | 71 ± 5 |
| GO | −36 ± 3 |
| GO_MD10 | 35 ± 5 |
| GR | −30 ± 3 |
| GR_MD10 | 61 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachá, D.; Muñoz-Bonilla, A.; Škrlová, K.; Echeverria, C.; Chiloeches, A.; Petr, M.; Lafdi, K.; Fernández-García, M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials 2020, 10, 1218. https://doi.org/10.3390/nano10061218
Plachá D, Muñoz-Bonilla A, Škrlová K, Echeverria C, Chiloeches A, Petr M, Lafdi K, Fernández-García M. Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials. Nanomaterials. 2020; 10(6):1218. https://doi.org/10.3390/nano10061218
Chicago/Turabian StylePlachá, Daniela, Alexandra Muñoz-Bonilla, Kateřina Škrlová, Coro Echeverria, Alberto Chiloeches, Martin Petr, Khalid Lafdi, and Marta Fernández-García. 2020. "Antibacterial Character of Cationic Polymers Attached to Carbon-Based Nanomaterials" Nanomaterials 10, no. 6: 1218. https://doi.org/10.3390/nano10061218