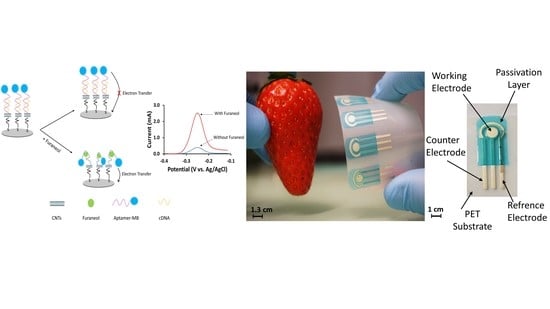
Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of the Aptasensor
2.2.1. Printing the Electrodes
2.2.2. Preparation of Nanomaterials
2.2.3. Stepwise Fabrication Strategy
CNT-Modified Electrode (CNT-ME)
AgNPs Modified Electrode (AgNP-ME)
2.3. Electrochemical Characterization of Furaneol Aptasensor
2.4. Optical Characterization
2.5. Detection of Furaneol
2.6. Mechanical Characterization
2.7. Detection of Furaneol in a Real Sample and Validation with HPLC
2.7.1. Characterization of Fruit Maturation Stages
2.7.2. Sample Preparation
2.7.3. HPLC Analysis
3. Results and Discussion
3.1. Working Principle of the Aptasensor
3.2. Characterization of Stepwise Fabrication of the Aptasensor
3.2.1. Surface Characterization
3.2.2. Electrochemical Characterization
3.2.3. FTIR Characterization
3.3. Aptasensor Performance Comparison
3.3.1. Electroactive area Evaluation of the CNT-ME and AgNP-ME
3.3.2. Detection Performance
Optimization of Experimental Conditions
Comparison between AgNP-ME and CNT-ME Aptasensor
3.4. CNT-ME Aptasensor
3.4.1. Analytical Performance of the Aptasensor
3.4.2. Repeatability
3.4.3. Storage Stability and Regeneration Test
3.4.4. Selectivity
3.4.5. Mechanical Stability Test
3.4.6. Detection of Furaneol in Real Samples and HPLC Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwab, W. Natural 4-hydroxy-2,5-dimethyl-3(2h)-furanone. Molecules 2013, 18, 6936–6951. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Ogawa, K.; Li, X.; Kikugawa, K. Inhibition of iron ion-induced oxidative damage of erythrocyte membranes and low density lipoprotein by a Maillard product.; 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone (HEMF). Biol. Pharm. Bull. 2000, 23, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiramoto, K.; Sekiguchi, K.; Ayuha, K.; Aso-o, R.; Moriya, N.; Kato, T.; Kikugawa, K. DNA breaking activity and mutagenicity of soy sauce: Characterization of the active components and identification of 4-hydroxy-5-methyl-3(2 H)-furanone. Mutat. Res. 1996, 359, 119–132. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Makino, T.; Yoshino, M. Prooxidant action of furanone compounds: Implication of reactive oxygen species in the metal-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Food Chem. Toxicol. 2007, 457, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Oey, I.; Lille, M.; Van Loey, A.; Hendrickx, M. Effect of high-pressure processing on color, texture and flavor of fruit- and vegetable-based food products: A review. Trends Food Sci. Technol. 2008, 19, 320–328. [Google Scholar] [CrossRef]
- Zabetakis, I.; Gramshaw, J.; Robinson, D. 2,5-Dimethyl-4-hydroxy-2H-furan-3-one and its derivatives: Analysis, synthesis and biosynthesis—A review. Food Chem. 1999, 65, 139–151. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Separation and Identification of Furanic Compounds in Fruit Juices and Drinks by High-Performance Liquid Chromatography Photodiode Array Detection. J. Agric. Food Chem. 1998, 46, 1286–1291. [Google Scholar] [CrossRef]
- Groyne, J.; Lognay, G.; Marlier, M. Accumulation of glycosidically bound compounds in Fragaria ×ananassa cv.Elsanta fruits at various developmental stages. Biotechnol. Agron. Soc. Environ. 1999, 3, 5–9. [Google Scholar]
- Buttery, R.G.; Takeoka, G.R.; Naim, M.; Rabinowitch, H.; Nam, Y. Analysis of Furaneol in tomato using dynamic headspace sampling with sodium sulfate. J. Agric. Food Chem. 2001, 49, 4349–4351. [Google Scholar] [CrossRef]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Vreuls, R.J.; Van der Heijden, A.; Brinkman, U.A.T. Adahchour, M. Trace-level determination of polar flavor compounds in butter by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 1999, 844, 295–305. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Tòth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Zare, H.; Evazalipour, M.; Mosaferg, J.; Tehrani, B.S.; Pasdar, A.; Mokhtarzadeh, A.; Ramezani, M. Aptasensors as a new sensing technology developed for the detection of MUC1 mucin: A review. Biosens. Bioelectron. 2019, 130, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Lee, C.S.; Kim, T.H. Electrochemical Detection of Ultratrace Lead Ion through Attaching and Detaching DNA Aptamer from Electrochemically Reduced Graphene Oxide Electrode. Nanomaterials 2019, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, F.; Abbaszadeh, H.; Dolatabadi, J.E.N.; Aghebati-Maleki, L.; Yousefi, M. Label-Free Electrochemical Aptasensor for Sensitive Detection of Malachite Green Based on Au Nanoparticle/Graphene Quantum Dots/Tungsten Disulfide Nanocomposites. Nanomaterials 2019, 9, 229. [Google Scholar]
- Kim, S.H.; Thoa, T.T.T.; Gu, M.B. Aptasensors for environmental monitoring of contaminants in water and soil. Curr. Opin. Environ. Sci. Health 2019, 10, 9–21. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef] [Green Version]
- Komarova, N.; Andrianova, M.; Glukhov, S.; Kuznetsov, A. Selection, Characterization, and Application of ssDNA Aptamer against Furaneol. Molecules 2018, 23, 3159. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, D.; Hachiya, N.H.; Kaneko, K.; Sode, K.; Ikebukuro, K. Detection system based on the conformational change in an aptamer and its application to simple bound/free separation. Biosens. Bioelectron. 2009, 24, 1372–1376. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Huanga, Y.; Yan, X.; Zhao, L.; Qi, X.; Wang, S.; Liang, X. An aptamer cocktail-based electrochemical aptasensor for direct capture and rapid detection of tetracycline in honey. Microchem. J. 2019, 150, 104179. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2019, 147, 111777. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, T.; Ramaraj, R. Simultaneous detection of ascorbic acid, uric acid and homovanillic acid at copper modified electrode. Electrochim. Acta 2007, 52, 2998–3005. [Google Scholar] [CrossRef]
- Rosati, G.; Ravarotto, M.; Scaramuzza, M.; De Toni, A.; Paccagnella, A. Silver nanoparticles inkjet-printed flexible biosensor for rapid label-free antibiotic detection in milk. Sens. Actuators B 2019, 208, 280–289. [Google Scholar] [CrossRef]
- Lee, K.; Yoo, Y.K.; Chae, M.; Hwang, K.S.; Lee, J.; Kim, H.; Hur, D. Highly selective reduced graphene oxide (rGO) sensor based on a peptide aptamer receptor for detecting explosives. Sci. Rep. 2019, 9, 10297. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Sh, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Falco, A.; Cina, L.; Scarpa, G.; Lugli, P.; Abdellah, A. Fully-Sprayed and Flexible Organic Photodiodes with Transparent. ACS Appl. Mater. Interfaces 2014, 6, 10593–10601. [Google Scholar] [CrossRef]
- Pérez, A.G.; Olías, R.; Sanz, C.; Olías, J.M. Furanones in Strawberries: Evolution during Ripening and Postharvest Shelf Life. J. Agric. Food Chem. 1996, 44, 3620–3624. [Google Scholar]
- Zhu, L.X.; Zhang, M.M.; Liu, Z.; Shi, Y.; Duan, C.Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation during Msalais Winemaking. Molecules 2019, 24, 3104. [Google Scholar] [CrossRef] [Green Version]
- Denga, B.; Lin, Y.; Wang, C.; Li, F.; Wang, Z.; Zhang, H.; Li, X.F.; Le, X.C. Aptamer binding assays for proteins: The thrombin example—A review. Anal. Chim. Acta 2014, 837, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.; Mukherjiabc, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutak, W.; Chhowalla, M.; Sesti, S. The chemical and physical characteristicsof single-walled carbon nanotube filmimpact on osteoblastic cell response. Nanotechnology 2010, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.; Akmal, M.R.; Salek-Maghsoudi, A.; Rahmani, S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Novel label-free electrochemical aptasensor for determination of Diazinon using gold nanoparticles-modified screen-printed gold electrode. Biosens. Bioelectron. 2018, 120, 122–128. [Google Scholar] [CrossRef]
- Islam Khan, N.I.; Maddaus, A.G.; Song, E. A Low-Cost Inkjet-Printed Aptamer-Based Electrochemical Biosensor for the Selective Detection of Lysozyme. Biosensors 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.; Ben Aissa, S.; Sherazi, T.A.; Catanante, G.; Hayat, A.; Marty, J.L. Development of an Impedimetric Aptasensor for Label Free Detection of Patulin in Apple Juice. Molecules 2019, 24, 1017. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lia, Y.; Zhao, Q. A signal-on electrochemical aptasensor for rapid detection of aflatoxin B1 based on competition with complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance Biosensors: Applications to Sustainability and Remaining Technical Challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef]
- Lipfert, J.; Doniach, S.; Das, R.; Herschlag, D. Understanding Nucleic Acid–Ion Interactions. Annu. Rev. Biochem. 2014, 83, 813–841. [Google Scholar] [CrossRef] [Green Version]
- Zamiri, R.; Ahangar, H.A.; Zakaria, A.; Zamiri, G.; Shabani, M.; Singh, B.K.; Ferreira, J. The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateishi-Karimata, H.; Sugimoto, N. Structure, stability and behaviour of nucleic acids in ionic liquids. Nucleic Acids Res. 2014, 42, 8831–8844. [Google Scholar] [CrossRef]
- Ghatak, B.; Naskar, H.; Babar-Ali, S.K.; Tudu, B.; Paramanik, P.; Mukherji, S.; Bandyopadhyay, R. Development of Furaneol Imprinted Polymer Based QCM sensor for Discrimination of Artificially and Naturally Ripened Mango. In Proceedings of the IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar]
- Xiao, Y.; Lai, R.Y.; Plaxco, K.W. Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat. Protoc. 2007, 2, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Bognár, Z.; Gyurcsányi, R.E. Finding the Optimal Surface Density of Aptamer Monolayers by SPR Imaging Detection-based Aptamer Microarrays. Electroanalysis 2020, 32, 851–858. [Google Scholar] [CrossRef]
- Bariya, M.; Shahpar, Z.; Park, H.; Sun, J.; Jung, Y.; Gao, W.; Nyein, H.Y.Y.; Liaw, T.S.; Tai, L.C.; Ngo, Q.P.; et al. Roll-to-Roll Gravure Printed Electrochemical. ACS Nano 2018, 12, 6978–6987. [Google Scholar] [CrossRef]







| Sensor | Biorecognition Element | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Field Effect Transistor | Aptamers | 0.1–10 µM | 0.1 µM | [19] |
| Quartz crystal microbalance | Molecular imprinted polymer | 780.46–7804.57 µM | 9.6 µM | [43] |
| Square Wave Voltammetry | Aptamers | 1 fM–35 µM | 0.557 fM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douaki, A.; Demelash Abera, B.; Cantarella, G.; Shkodra, B.; Mushtaq, A.; Ibba, P.; Inam, A.S.; Petti, L.; Lugli, P. Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance. Nanomaterials 2020, 10, 1167. https://doi.org/10.3390/nano10061167
Douaki A, Demelash Abera B, Cantarella G, Shkodra B, Mushtaq A, Ibba P, Inam AS, Petti L, Lugli P. Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance. Nanomaterials. 2020; 10(6):1167. https://doi.org/10.3390/nano10061167
Chicago/Turabian StyleDouaki, Ali, Biresaw Demelash Abera, Giuseppe Cantarella, Bajramshahe Shkodra, Asma Mushtaq, Pietro Ibba, AKM Sarwar Inam, Luisa Petti, and Paolo Lugli. 2020. "Flexible Screen Printed Aptasensor for Rapid Detection of Furaneol: A Comparison of CNTs and AgNPs Effect on Aptasensor Performance" Nanomaterials 10, no. 6: 1167. https://doi.org/10.3390/nano10061167