Highly Catalytic Electrochemical Oxidation of Carbon Monoxide on Iridium Nanotubes: Amperometric Sensing of Carbon Monoxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ir Metal Nanotubes
2.3. Physical Characterization
2.4. Electrode Preparation and Electrochemical Measurements
2.5. DFT Calculation
3. Results and Discussion
3.1. Synthetic Condition and Physical Characterization of IrO2 and Ir Metal Nanotubes
3.2. Electrochemical Characterization of the Nanotubes for CO Oxidation
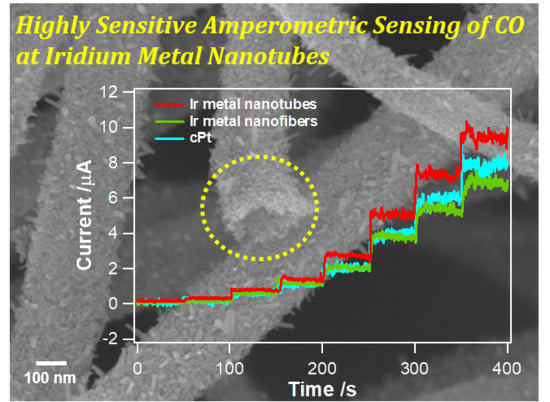
3.3. Amperometric Responses to CO at Ir Metal Nanotubes
3.4. DFT Calculations for the CO Adsorption Energy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jin, Y.; Guo, L.; Veiga, M.C.; Kennes, C. Optimization of the treatment of carbon monoxide-polluted air in biofilters. Chemosphere 2009, 74, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas sensors: A review. Sens. Transducers J. 2014, 168, 61–75. [Google Scholar]
- Van der Wal, P.D.; de Rooij, N.F.; Koudelka-Hep, M. Extremely stable nafion based carbon monoxide sensor. Sens. Actuators B 1996, 35, 119–123. [Google Scholar] [CrossRef]
- Chen, C.; He, J.; Xu, D.; Tan, X.; Zhou, X.; Wang, X. Study of nano-Au-assembled amperometric CO gas sensor. Sens. Actuators B 2005, 107, 866–871. [Google Scholar] [CrossRef]
- Rodriguez, P.; Koper, M.T.M. Electrocatalysis on gold. Phys. Chem. Chem. Phys. 2014, 16, 13583–13594. [Google Scholar] [CrossRef] [PubMed]
- Hayden, B.E.; Pletcher, D.; Suchsland, J.-P. Enhanced activity for electrocatalytic oxidation of carbon monoxide on titania-supported gold nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 3530–3532. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gasteiger, H.A.; Markovic, N.M.; Ross, P.N. On the reaction pathway for methanol and carbon monoxide electrooxidation on Pt-Sn alloy versus Pt-Ru alloy surfaces. Electrochim. Acta 1996, 41, 2587–2593. [Google Scholar] [CrossRef]
- Santhosh, P.; Manesh, K.M.; Gopalan, A.; Lee, K.-P. Novel amperometric carbon monoxide sensor based on multi-wall carbon nanotubes grafted with polydiphenylamine—Fabrication and performance. Sens. Actuators B 2007, 125, 92–99. [Google Scholar] [CrossRef]
- Yasuda, A.; Shimidzu, T. Electrochemical carbon monoxide sensor with a Nafion® film. React. Funct. Polym. 1999, 41, 235–243. [Google Scholar] [CrossRef]
- Li, X.; Xuan, T.; Yin, G.; Gao, Z.; Zhao, K.; Yan, P.; He, D. Highly sensitive amperometric CO sensor using nanocomposite C-loaded PdCl2–CuCl2 as sensing electrode materials. J. Alloys Compd. 2015, 645, 553–558. [Google Scholar] [CrossRef]
- Tsceng, K.-I.; Yang, M.-C. Platinum electrodes modified by tin for electrochemical CO sensors. J. Electrochem. Soc. 2003, 150, H156–H160. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Liu, F.; Wang, B.; Yang, X.; Liang, X.; Suo, H.; Sun, P.; Sun, Y.; Ma, J.; Zheng, J. Highly sensitive amperometric Nafion-based CO sensor using Pt/C electrodes with different kinds of carbon materials. Sens. Actuators B 2017, 239, 696–703. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Liang, H.-W.; Liu, S.; Yu, S.-H. Controlled synthesis of one-dimensional inorganic nanostructures using pre-existing one-dimensional nanostructures as templates. Adv. Mater. 2010, 22, 3925–3937. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Cheng, H.; Xie, W.; Sun, L. Effects of diameter and hollow structure on the microwave absorption properties of short carbon fibers. Ceram. Int. 2012, 38, 4867–4873. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Davoudpour, Y.; Habibi, Y.; Elbahri, M. The electrospun ceramic hollow nanofibers. Nanomaterials 2017, 7, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.-C.; Chen, Y.-C.; Huang, C.-N. Free-standing iridium oxide nanotube array for neural interface electrode applications. Mater. Lett. 2018, 221, 293–295. [Google Scholar] [CrossRef]
- Mafakheri, E.; Salimi, A.; Hallaj, R.; Ramazani, A.; Kashi, M.A. Synthesis of iridium oxide nanotubes by electrodeposition into polycarbonate template: Fabrication of chromium(III) and arsenic(III) electrochemical sensor. Electroanalysis 2011, 23, 2429–2437. [Google Scholar] [CrossRef]
- Chen, R.-S.; Huang, Y.-S.; Tsai, D.-S.; Chattopadhyay, S.; Wu, C.-T.; Lan, Z.-H.; Chen, K.-H. Growth of well aligned IrO2 nanotubes on LiTaO3(012) substrate. Chem. Mater. 2004, 16, 2457–2462. [Google Scholar] [CrossRef]
- Geiger, S.; Kasian, O.; Shrestha, B.R.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. Activity and stability of electrochemically and thermally treated iridium for the oxygen evolution reaction. J. Electrochem. Soc. 2016, 163, F3132–F3138. [Google Scholar] [CrossRef] [Green Version]
- Antolini, E. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jung, H.; Lee, C.; Kim, M.H.; Lee, Y. Comparative study on hydrogen evolution reaction activity of electrospun nanofibers with diverse metallic Ir and IrO2 composition ratios. ACS Sustain. Chem. Eng. 2019, 7, 8613–8620. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, Y.L.; Yu, A.; Lee, J.; Lee, S.C.; Lee, C.; Kim, M.H.; Lee, Y. Electrospun iridium oxide nanofibers for direct selective electrochemical detection of ascorbic acid. Sens. Actuators B 2014, 196, 480–488. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J. Simultaneous electrochemical detection of nitric oxide and carbon monoxide generated from mouse kidney organ tissues. Anal. Chem. 2007, 79, 7669–7675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Wang, X.; Chen, Y.; Zhou, Y.; Tang, Y.; Lu, L.; Bao, J.; Lu, T. Preparation of Pd–Au/C catalysts with different alloying degree and their electrocatalytic performance for formic acid oxidation. Appl. Catal. B 2011, 102, 614–619. [Google Scholar] [CrossRef]
- Moon, S.; Cho, Y.-B.; Yu, A.; Kim, M.H.; Lee, C.; Lee, Y. Single-step electrospun Ir/IrO2 nanofibrous structures decorated with Au nanoparticles for highly catalytic oxygen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 1979–1987. [Google Scholar] [CrossRef]
- Li, L.; Peng, S.; Cheah, Y.; Teh, P.; Wang, J.; Wee, G.; Ko, Y.; Wong, C.; Srinivasan, M. Electrospun porous NiCo2O4 nanotubes as advanced electrodes for electrochemical capacitors. Chem. Eur. J. 2013, 19, 5892–5898. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yu, A.; Lee, Y.; Kim, H.Y.; Kim, Y.J.; Lee, N.-S.; Lee, C.; Lee, Y.; Kim, M.H. Single phase of spinel Co2RhO4 nanotubes with remarkably enhanced catalytic performance for the oxygen evolution reaction. Nanoscale 2019, 11, 9287–9295. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical methods of real surface area determination of noble metal electrodes—An overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Orita, H.; Itoh, N.; Inada, Y. All electron scalar relativistic calculations on adsorption of CO on Pt(111) with full-geometry optimization: A correct estimation for CO site-preference. Chem. Phys. Lett. 2004, 384, 271–276. [Google Scholar] [CrossRef]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef] [PubMed]
- McPherson, I.J.; Ash, P.A.; Jones, L.; Varambhia, A.; Jacobs, R.M.J.; Vincent, K.A. Electrochemical CO oxidation at platinum on carbon studied through analysis of anomalous in situ IR spectra. J. Phys. Chem. C 2017, 121, 17176–17187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Gómez, R.; Weaver, M.J. Nitric oxide and carbon monoxide adsorption on polycrystalline iridium electrodes: A combined raman and infrared spectroscopic study. Langmuir 1997, 13, 6713–6721. [Google Scholar] [CrossRef]
- Zhang, C.J.; Baxter, R.J.; Hu, P.; Alavi, A.; Lee, M.H. A density functional theory study of carbon monoxide oxidation on the Cu3Pt(111) alloy surface: Comparison with the reactions on Pt(111) and Cu(111). J. Chem. Phys. 2001, 115, 5272–5277. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Kwon, T.; Lee, C.; Lee, Y. Highly Catalytic Electrochemical Oxidation of Carbon Monoxide on Iridium Nanotubes: Amperometric Sensing of Carbon Monoxide. Nanomaterials 2020, 10, 1140. https://doi.org/10.3390/nano10061140
Yu A, Kwon T, Lee C, Lee Y. Highly Catalytic Electrochemical Oxidation of Carbon Monoxide on Iridium Nanotubes: Amperometric Sensing of Carbon Monoxide. Nanomaterials. 2020; 10(6):1140. https://doi.org/10.3390/nano10061140
Chicago/Turabian StyleYu, Areum, Taehui Kwon, Chongmok Lee, and Youngmi Lee. 2020. "Highly Catalytic Electrochemical Oxidation of Carbon Monoxide on Iridium Nanotubes: Amperometric Sensing of Carbon Monoxide" Nanomaterials 10, no. 6: 1140. https://doi.org/10.3390/nano10061140