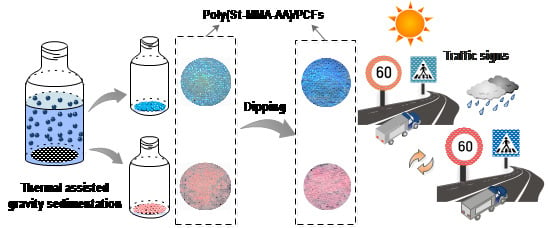
Fabrication of Structural-Coloured Carbon Fabrics by Thermal Assisted Gravity Sedimentation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Purification of Poly(St-MMA-AA) Nanoparticles
2.3. Generation of Poly(St-MMA-AA) Photonic Crystal Films on Carbon Fabrics
2.4. Characterisation Methods
2.4.1. Water-in-Air Contact Angle (WCA) Measurement
2.4.2. Microscopic Observation
2.4.3. Particle Size and Polydispersity Indices (Ð) Measurement
2.4.4. Optical Image Acquisition
2.4.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.6. Raman Spectroscopy
2.4.7. Colour Property Measurement
2.4.8. Electrical Resistivity Measurement
3. Results and Discussion
3.1. WCA Analysis of Carbon Fabrics
3.2. TEM Observation and Particle Size Analysis
3.3. Chemical Characteristics by FT-IR and Raman Spectroscopy
3.4. Colour Measurement
3.5. Electrical Resistivity of Poly(St-MMA-AA)/PCFs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Li, Y.; Liu, G.; Fan, Q.; Shao, J. Study on the correlations between the structural colors of photonic crystals and the base colors of textile fabric substrates. Dyes Pigm. 2016, 133, 435–444. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, L.; Zhang, G.; Li, Y.; Chai, L.; Fan, Q.; Shao, J. Fabrication of patterned photonic crystals with brilliant structural colors on fabric substrates using ink-jet printing technology. Mater. Des. 2017, 114, 10–17. [Google Scholar] [CrossRef]
- Ge, D.; Lee, E.; Yang, L.; Cho, Y.; Li, M.; Gianola, D.S.; Yang, S. A robust smart window: reversibly switching from high transparency to angle-independent structural color display. Adv. Mater. 2015, 27, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Shim, T.S.; Hwang, H.; Yang, S.M.; Kim, S.H. Colloidal Photonic Crystals toward Structural Color Palettes for Security Materials. Chem. Mater. 2013, 25, 2684–2690. [Google Scholar] [CrossRef]
- Zhou, C.; Qi, Y.; Zhang, S.; Niu, W.; Ma, W.; Wu, S.; Tang, B. Rapid fabrication of vivid noniridescent structural colors on fabrics with robust structural stability by screen printing. Dyes Pigm. 2020, 176, 108226. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Shi, L.; Qiu, H.; Zhang, S.; Qi, N.; Hu, J.; Yuan, W.; Zhang, X.; Zhang, K.Q. Additive Mixing and Conformal Coating of Noniridescent Structural Colors with Robust Mechanical Properties Fabricated by Atomization Deposition. ACS Nano 2018, 12, 3095–3102. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, C.; Tian, L.; Liu, C.; Fu, G.; Shang, L.; Gu, Z. Structural Color Patterns by Electrohydrodynamic Jet Printed Photonic Crystals. ACS Appl. Mater. Interfaces 2017, 9, 11933–11941. [Google Scholar] [CrossRef]
- Chen, S.; Su, M.; Zhang, C.; Gao, M.; Bao, B.; Yang, Q.; Su, B.; Song, Y. Fabrication of Nanoscale Circuits on Inkjet-Printing Patterned Substrates. Adv. Mater. 2015, 27, 3928–3933. [Google Scholar] [CrossRef]
- Zeng, Q.; Ding, C.; Li, Q.; Yuan, W.; Peng, Y.; Hu, J.; Zhang, K.Q. Rapid fabrication of robust, washable, self-healing superhydrophobic fabrics with non-iridescent structural color by facile spray coating. RSC Adv. 2017, 7, 8443–8452. [Google Scholar] [CrossRef] [Green Version]
- Ishii, M.; Narita, T.; Hayata, Y.; Nishimura, A.; Tachi, K. Simple and Rapid Fabrication of Large-Scale Polymer-Immobilized Colloidal Crystals by Spray Coating. Macromol. Mater. Eng. 2011, 296, 687–692. [Google Scholar] [CrossRef]
- Gao, W.; Rigout, M.; Owens, H. Self-assembly of silica colloidal crystal thin films with tuneable structural colours over a wide visible spectrum. Appl. Surf. Sci. 2016, 380, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhou, L.; Wang, C.; Wu, Y.; Li, Y.; Fan, Q.; Shao, J. Study on the high hydrophobicity and its possible mechanism of textile fabric with structural colors of three-dimensional poly(styrene-methacrylic acid) photonic crystals. RSC Adv. 2015, 5, 62855–62863. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Wang, H.; Li, Y. Structurally colored carbon fibers with controlled optical properties prepared by a fast and continuous electrophoretic deposition method. Nanoscale 2013, 5, 6917–6922. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, K.; Tanaka, Y.; Uemura, K.; Inumaru, K.; Seki, T.; Takeoka, Y. Structural color coating films composed of an amorphous array of colloidal particles via electrophoretic deposition. NPG Asia Mater. 2017, 9, e355. [Google Scholar] [CrossRef]
- Wu, X.; Hong, R.; Meng, J.; Cheng, R.; Zhu, Z.; Wu, G.; Li, Q.; Wang, C.F.; Chen, S. Hydrophobic Poly(tert-butyl acrylate) Photonic Crystals towards Robust Energy-Saving Performance. Angew. Chem. Int. Ed. Engl. 2019, 58, 13556–13564. [Google Scholar] [CrossRef]
- Fan, W.; Zeng, J.; Gan, Q.; Ji, D.; Song, H.; Liu, W.; Shi, L.; Wu, L. Iridescence-controlled and flexibly tunable retroreflective structural color film for smart displays. Sci. Adv. 2019, 5, eaaw8755. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Qiu, J.; Wu, S.; Ju, B.; Zhang, S.; Tang, B. Biomimetic Structural Color Films with a Bilayer Inverse Heterostructure for Anticounterfeiting Applications. ACS Appl. Mater. Interfaces 2018, 10, 38459–38465. [Google Scholar] [CrossRef]
- Guo, J.; Li, H.; Ling, L.; Li, G.; Cheng, R.; Lu, X.; Xie, A.Q.; Li, Q.; Wang, C.F.; Chen, S. Green Synthesis of Carbon Dots toward Anti-Counterfeiting. ACS Sustain. Chem. Eng. 2020, 8, 1566–1572. [Google Scholar] [CrossRef]
- Kats, M.A.; Blanchard, R.; Genevet, P.; Capasso, F. Nanometre optical coatings based on strong interference effects in highly absorbing media. Nat. Mater. 2013, 12, 20–24. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Cui, H.; Zhao, Q.; Chen, H.; He, L.; Wang, Y. Breath-Taking Patterns: Discontinuous Hydrophilic Regions for Photonic Crystal Beads Assembly and Patterns Revisualization. ACS Appl. Mater. Interfaces 2017, 9, 38117–38124. [Google Scholar] [CrossRef]
- Gallei, M. Functional Polymer Opals and Porous Materials by Shear-Induced Assembly of Tailor-Made Particles. Macromol. Rapid Commun. 2018, 39, 1700648. [Google Scholar] [CrossRef]
- Finlayson, C.E.; Baumberg, J.J. Generating bulk-scale ordered optical materials using shear-assembly in viscoelastic media. Materials 2017, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Finlayson, C.E.; Snoswell, D.R.; Haines, A.; Schäfer, C.; Spahn, P.; Hellmann, G.P.; Petukhov, A.V.; Herrmann, L.; Burdet, P.; et al. Large-scale ordering of nanoparticles using viscoelastic shear processing. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pursiainen, O.L.; Baumberg, J.J.; Winkler, H.; Viel, B.; Spahn, P.; Ruhl, T. Nanoparticle-tuned structural color from polymer opals. Opt. Express 2007, 15, 9553–9561. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Gallei, M.; Zahn, J.T.; Engelhardt, J.; Hellmann, G.P.; Rehahn, M. Reversible light-, thermo-, and mechano-responsive elastomeric polymer opal films. Chem. Mater. 2013, 25, 2309–2318. [Google Scholar] [CrossRef]
- Ding, T.; Smoukov, S.K.; Baumberg, J.J. Stamping colloidal photonic crystals: a facile way towards complex pixel colour patterns for sensing and displays. Nanoscale 2015, 7, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Qi, N.; Peng, Y.; Zhang, Y.; Shi, L.; Zhang, X.; Lai, Y.; Wei, K.; Kim, I.S.; Zhang, K.-Q. Sub-micron silk fibroin film with high humidity sensibility through color changing. RSC Adv. 2017, 7, 17889–17897. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, G.; Felgueiras, H.P.; Ribeiro, A.I.; Seventekin, N.; Zille, A.; Souto, A.P. Dyed Poly(styrene-methyl Methacrylate-acrylic Acid) Photonic Nanocrystals for Enhanced Structural Color. ACS Appl. Mater. Interfaces 2018, 10, 23285–23294. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, J.; Tong, Y.-l.; Peng, G.; Cui, T.; Wang, C.-F.; Chen, S.; Weitz, D.A. Reduced Graphene Oxide Membrane Induced Robust Structural Colors toward Personal Thermal Management. ACS Photonics 2018, 6, 116–122. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, N.; Shi, L.; Zhang, K.Q. Structural Coloration of Colloidal Fiber by Photonic Band Gap and Resonant Mie Scattering. ACS Appl. Mater. Interfaces 2015, 7, 14064–14071. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Winter, T.; Heidt, S.; Dietz, C.; Ding, T.; Baumberg, J.J.; Gallei, M. Smart polymer inverse-opal photonic crystal films by melt-shear organization for hybrid core–shell architectures. J. Mater. Chem. C 2015, 3, 2204–2214. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, A.C.; Míguez, H.; Kitaev, V.; Ozin, G.A.; Manners, I. A Polychromic, Fast Response Metallopolymer Gel Photonic Crystal with Solvent and Redox Tunability: A Step Towards Photonic Ink (P-Ink). Adv. Mater. 2003, 15, 503–507. [Google Scholar] [CrossRef]
- Winter, T.; Su, X.; Hatton, T.A.; Gallei, M. Ferrocene-Containing Inverse Opals by Melt-Shear Organization of Core/Shell Particles. Macromol. Rapid Commun. 2018, 39, 1800428. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Arsenault, A.C.; Manners, I.; Ozin, G.A. Electroactive inverse opal: a single material for all colors. Angew. Chem. Int. Ed. 2009, 48, 943–947. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z. Gated graphene island-enabled tunable charge transfer plasmon terahertz metamodulator. Nanoscale 2019, 11, 8091–8095. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Ahmadivand, A.; Pala, N. Hybridized plasmons in graphene nanorings for extreme nonlinear optics. Opt. Mater. 2017, 73, 729–735. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Noe, G.T.; Mishra, Y.K. Gated graphene enabled tunable charge–current configurations in hybrid plasmonic metamaterials. ACS Appl. Electron. Mater. 2019, 1, 637–641. [Google Scholar] [CrossRef]
- Shang, S.; Liu, Z.; Zhang, Q.; Wang, H.; Li, Y. Facile fabrication of a magnetically induced structurally colored fiber and its strain-responsive properties. J. Mater. Chem. A 2015, 3, 11093–11097. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, A.; Shi, L.; Zhang, K.Q. Fabrication of Structurally-Colored Fibers with Axial Core–Shell Structure via Electrophoretic Deposition and Their Optical Properties. ACS Macro Lett. 2013, 2, 116–120. [Google Scholar] [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Sporl, J.M.; Buchmeiser, M.R. Carbon fibers: precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. Engl. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef] [Green Version]
- Cong, H.; Cao, W. Colloidal Crystallization Induced by Capillary Force. Langmuir 2003, 19, 8177–8181. [Google Scholar] [CrossRef]
- Fitch, R.M.; Prenosil, M.B.; Sprick, K.J. The mechanism of particle formation in polymer hydrosols. I. Kinetics of Aqueous Polymerization of Methyl Methacrylate. J. Polym. Sci. Part. C Polym. Symp. 1969, 27, 95–118. [Google Scholar] [CrossRef]
- Fitch, R.M.; Tsai, C.H. Polymer Colloids; Plenum Press: New York, NY, USA, 1971; p. 73. [Google Scholar]
- Aslamazova, T.R. Emulsifier-free latexes and polymers on their base. Prog. Org. Coat. 1995, 25, 109–167. [Google Scholar] [CrossRef]
- Litt, M.; Patsiga, R.; Stannett, V. Emulsion polymerization of vinyl acetate. II. Polymer Chemistry. J. Polym. Sci. Part A -1 1970, 8, 3607–3649. [Google Scholar] [CrossRef]
- Hansen, F.K.; Ugelstad, J. Particle nucleation in emulsion polymerization. III. Nucleation in systems with anionic emulsifier investigated by seeded and unseeded polymerization. J. Poly. Sci. Polym. Chem. Ed. 1979, 17, 3047–3067. [Google Scholar] [CrossRef]
- Willis, H.A.; Zichy, V.J.I.; Hendra, P.J. The laser-Raman and infra-red spectra of poly (methyl methacrylate). Polymer 1969, 10, 737–746. [Google Scholar] [CrossRef]
- Ifijen, H.I.; Ikhuoria, E.U.; Omorogbe, S.O. Correlative studies on the fabrication of poly(styrene-methyl-methacrylate-acrylic acid) colloidal crystal films. J. Dispers. Sci. Technol. 2019, 40, 1023–1030. [Google Scholar] [CrossRef]
- Thakur, V.K.; Vennerberg, D.; Madbouly, S.A.; Kessler, M.R. Bio-inspired green surface functionalization of PMMA for multifunctional capacitors. RSC Adv. 2014, 4, 6677–6684. [Google Scholar] [CrossRef] [Green Version]
- Olmos, D.; Martin, E.V.; Gonzalez-Benito, J. New molecular-scale information on polystyrene dynamics in PS and PS-BaTiO3 composites from FTIR spectroscopy. PCCP 2014, 16, 24339–24349. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, W.; Qu, B. Structural characterisation and thermal properties of exfoliated polystyrene/ZnAl layered double hydroxide nanocomposites prepared via solution intercalation. Polym. Degrad. Stab. 2005, 87, 433–440. [Google Scholar] [CrossRef]
- Gu, R.; Xu, W.Z.; Charpentier, P.A. Synthesis of graphene-polystyrene nanocomposites via RAFT polymerization. Polymer 2014, 55, 5322–5331. [Google Scholar] [CrossRef]
- León-Bermúdez, A.Y.; Salazar, R. Synthesis and characterization of the polystyrene-asphaltene graft copolymer by FT-IR spectroscopy. CT&F-Ciencia Tecnología y Futuro 2008, 3, 157–167. [Google Scholar]
- Lai, C.F.; Li, J.S. Self-assembly of colloidal Poly(St-MMA-AA) core/shell photonic crystals with tunable structural colors of the full visible spectrum. Opt. Mater. 2019, 88, 128–133. [Google Scholar] [CrossRef]
- Watanabe, K.; Palonpon, A.F.; Smith, N.I.; Kasai, A.; Hashimoto, H.; Kawata, S.; Fujita, K. Structured line illumination Raman microscopy. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and near-infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly (acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Bridges, T.E.; Houlne, M.P.; Harris, J.M. Spatially resolved analysis of small particles by confocal Raman microscopy: Depth profiling and optical trapping. Anal. Chem. 2004, 76, 576–584. [Google Scholar] [CrossRef]
- Fan, Y.; Cornelius, C.J. Raman spectroscopic and gas transport study of a pentablock ionomer complexed with metal ions and its relationship to physical properties. J. Mater. Sci. 2013, 48, 1153–1161. [Google Scholar] [CrossRef]
- Mazilu, M.; De Luca, A.C.; Riches, A.; Herrington, C.S.; Dholakia, K. Optimal algorithm for fluorescence suppression of modulated Raman spectroscopy. Opt. Express 2010, 18, 11382–11395. [Google Scholar] [CrossRef]
- Xu, X.; Ming, H.; Zhang, Q.; Zhang, Y. Properties of Raman spectra and laser-induced birefringence in polymethyl methacrylate optical fibres. J. Opt. A Pure Appl. Opt. 2002, 4, 237–242. [Google Scholar]
- Zhang, Z.; Shen, W.; Ye, C.; Luo, Y.; Li, S.; Li, M.; Xu, C.; Song, Y. Large-area, crack-free polysilazane-based photonic crystals. J. Mater. Chem. 2012, 22, 5300–5303. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, J.; Wang, C.F.; Chen, S. Construction of Hydrogen-Bond-Assisted Crack-Free Photonic Crystal Films and Their Performance on Fluorescence Enhancement Effect. Macromol. Mater. Eng. 2017, 302, 1700013. [Google Scholar] [CrossRef]
- Hiltner, P.A.; Krieger, I.M. Diffraction of light by ordered suspensions. J. Phy. Chem. 1969, 73, 2386–2389. [Google Scholar] [CrossRef]
- Wang, M.; Meng, F.; Wu, H.; Wang, J. Photonic Crystals with an Eye Pattern Similar to Peacock Tail Feathers. Crystals 2016, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.H. Chameleon-inspired mechanochromic photonic films composed of non-close-packed colloidal arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Pointer, M.R. A comparison of the CIE 1976 colour spaces. Color. Res. Appl. 1981, 6, 108–118. [Google Scholar] [CrossRef]









| Particle size (nm) | L* 1 | a* 2 | a* | Colour shift related to a* | b* 3 | Colour shift related to b* | ΔEab* | ||
|---|---|---|---|---|---|---|---|---|---|
| 308.3 | 58.11 | 3.47 | −12.73 | Green shift | −4.07 | 6.44 | Yellow shift | 14.39 | |
| 262.4 | 59.96 | −9.26 | 2.32 | ||||||
| −10.78 | Green shift | −2.36 | Blue shift | 13.09 | |||||
| 249.7 | 52.92 | −20.04 | −0.04 | ||||||
| 9.86 | Red shift | −5.34 | Blue shift | 11.37 | |||||
| 234.1 | 54.9 | −10.18 | −5.38 | ||||||
| 7.49 | Red shift | 26.50 | Yellow shift | 28.27 | |||||
| 213.1 | 48.53 | −2.69 | −21.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Lee, C.H.; Kan, C.-W.; Jin, S. Fabrication of Structural-Coloured Carbon Fabrics by Thermal Assisted Gravity Sedimentation Method. Nanomaterials 2020, 10, 1133. https://doi.org/10.3390/nano10061133
Yu J, Lee CH, Kan C-W, Jin S. Fabrication of Structural-Coloured Carbon Fabrics by Thermal Assisted Gravity Sedimentation Method. Nanomaterials. 2020; 10(6):1133. https://doi.org/10.3390/nano10061133
Chicago/Turabian StyleYu, Jiali, Cheng Hao Lee, Chi-Wai Kan, and Shixin Jin. 2020. "Fabrication of Structural-Coloured Carbon Fabrics by Thermal Assisted Gravity Sedimentation Method" Nanomaterials 10, no. 6: 1133. https://doi.org/10.3390/nano10061133