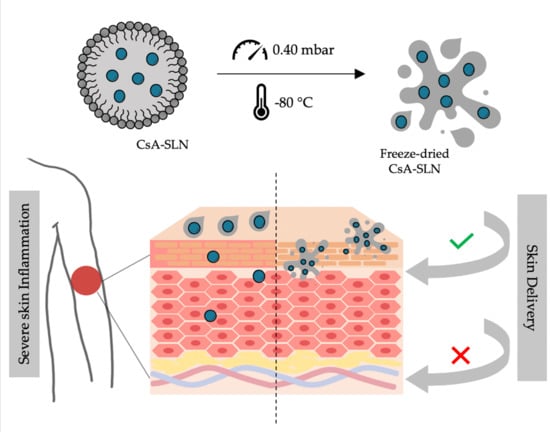
Freeze-Dried Softisan® 649-Based Lipid Nanoparticles for Enhanced Skin Delivery of Cyclosporine A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid Lipid Nanoparticles
2.3. Characterization of Solid Lipid Nanoparticles
2.3.1. Average Size and Surface Potential Determination
2.3.2. Morphology Assessment
2.3.3. Quantification of the Entrapment Efficiency and Drug Loading
2.3.4. Freeze-Drying
2.3.5. Fourier-Infrared Spectroscopy Evaluation
2.3.6. Storage Stability Studies
2.4. Rheological Properties
2.5. Cellular Studies
2.5.1. Cell Culture Conditions
2.5.2. Cell Viability Assays
2.5.3. Cell Uptake Assays
2.6. In Vitro Skin Permeation Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Softisan® 649/Tween® 80-Based Nanoparticles
3.2. Morphology Analysis
3.3. Fourier-Infrared Spectroscopy Evaluation
3.4. Assessment of the Storage Stability
3.5. Cellular Studies
3.5.1. Cell Viability
3.5.2. Softisan® 649/Tween® 80-Based Nanoparticles Internalization by Keratinocytes
3.6. Rheological Properties of Freeze-Dried Nanoformulations
3.7. In Vitro CsA Skin Permeation Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffiths, C.E.; van de Kerkhof, P.; Czarnecka-Operacz, M. Psoriasis and Atopic Dermatitis. Dermatol. Ther. 2017, 7, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.; Katelaris, C. Long-term management of patients taking immunosuppressive drugs. Aust. Prescr. 2009, 32, 68–71. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Dilbaghi, N.; Rani, R.; Bhanjana, G. Nanotechnology as Emerging Tool for Enhancing Solubility of Poorly Water-Soluble Drugs. BioNanoScience 2012, 2, 227–250. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Müller, R.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Kakadia, P.G.; Conway, B.R. Solid Lipid Nanoparticles: A Potential Approach for Dermal Drug Delivery. Am. J. Pharmacol. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lombardi Borgia, S.; Regehly, M.; Sivaramakrishnan, R.; Mehnert, W.; Korting, H.C.; Danker, K.; Roder, B.; Kramer, K.D.; Schafer-Korting, M. Lipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J. Control. Release 2005, 110, 151–163. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, S.; Herrmann, W.; Panek-Minkin, G.; Blaschke, T.; Zoschke, C.; Kramer, K.D.; Bittl, R.; Schafer-Korting, M. SLN for topical application in skin diseases-characterization of drug-carrier and carrier-target interactions. Int. J. Pharm. 2010, 390, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Schafer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Amor, K.T.; Ryan, C.; Menter, A. The use of cyclosporine in dermatology: Part I. J. Am. Acad. Dermatol. 2010, 63, 925–946. [Google Scholar] [CrossRef] [PubMed]
- Guada, M.; Beloqui, A.; Kumar, M.N.; Preat, V.; Dios-Vieitez Mdel, C.; Blanco-Prieto, M.J. Reformulating cyclosporine A (CsA): More than just a life cycle management strategy. J. Control. Release 2016, 225, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Amor, K.T.; Menter, A. The use of cyclosporine in dermatology: Part II. J. Am. Acad. Dermatol. 2010, 63, 949–972. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Nepovimova, E.; Wang, Y.; Yang, H.; Kuca, K. Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem. Toxicol. 2018, 118, 889–907. [Google Scholar] [CrossRef]
- Chen, M.; Kumar, S.; Anselmo, A.C.; Gupta, V.; Slee, D.H.; Muraski, J.A.; Mitragotri, S. Topical delivery of Cyclosporine A into the skin using SPACE-peptide. J. Control. Release 2015, 199, 190–197. [Google Scholar] [CrossRef]
- Frusic-Zlotkin, M.; Soroka, Y.; Tivony, R.; Larush, L.; Verkhovsky, L.; Bregegere, F.M.; Neuman, R.; Magdassi, S.; Milner, Y. Penetration and biological effects of topically applied cyclosporin A nanoparticles in a human skin organ culture inflammatory model. Exp. Dermatol. 2012, 21, 938–943. [Google Scholar] [CrossRef] [PubMed]
- El Tayar, N.; Mark, A.E.; Vallat, P.; Brunne, R.M.; Testa, B.; van Gunsteren, W.F. Solvent-dependent conformation and hydrogen-bonding capacity of cyclosporin A: Evidence from partition coefficients and molecular dynamics simulations. J. Med. Chem. 1993, 36, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, A. Oral cyclosporine A—The current picture of its liposomal and other delivery systems. Cell. Mol. Biol. Lett. 2009, 14, 139–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, L.B.; Collett, J.H.; Bentley, M.V. Topical delivery of cyclosporin A: An in vitro study using monoolein as a penetration enhancer. Eur. J. Pharm. Biopharm. 2005, 60, 25–30. [Google Scholar] [CrossRef]
- Varia, J.K.; Dodiya, S.S.; Sawant, K.K. Cyclosporine a loaded solid lipid nanoparticles: Optimization of formulation, process variable and characterization. Curr. Drug Deliv. 2008, 5, 64–69. [Google Scholar] [CrossRef]
- Kim, S.T.; Jang, D.J.; Kim, J.H.; Park, J.Y.; Lim, J.S.; Lee, S.Y.; Lee, K.M.; Lim, S.J.; Kim, C.K. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation. Pharmazie 2009, 64, 510–514. [Google Scholar] [CrossRef]
- Blanco-Prieto, M.J.; Guada, M.; Sebastian, V.; Irusta, S.; Feijoo, E.; Dios-Vieitez, M.d.C. Lipid nanoparticles for cyclosporine A administration: Development, characterization, and in vitro evaluation of their immunosuppression activity. Int. J. Nanomed. 2015, 10, 6541–6553. [Google Scholar] [CrossRef] [Green Version]
- Guada, M.; Beloqui, A.; Alhouayek, M.; Muccioli, G.G.; Dios-Vieitez Mdel, C.; Preat, V.; Blanco-Prieto, M.J. Cyclosporine A-loaded lipid nanoparticles in inflammatory bowel disease. Int. J. Pharm. 2016, 503, 196–198. [Google Scholar] [CrossRef]
- Guada, M.; Lana, H.; Gil, A.G.; Dios-Vieitez Mdel, C.; Blanco-Prieto, M.J. Cyclosporine A lipid nanoparticles for oral administration: Pharmacodynamics and safety evaluation. Eur. J. Pharm. Biopharm. 2016, 101, 112–118. [Google Scholar] [CrossRef]
- Guada, M.; Lasa-Saracibar, B.; Lana, H.; Dios-Vieitez Mdel, C.; Blanco-Prieto, M.J. Lipid nanoparticles enhance the absorption of cyclosporine A through the gastrointestinal barrier: In vitro and in vivo studies. Int. J. Pharm. 2016, 500, 154–161. [Google Scholar] [CrossRef]
- Aksungur, P.; Demirbilek, M.; Denkbas, E.; Vandervoort, J.; Ludwig, A.; Unlü, N. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake, and kinetic studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mittal, A.; Jain, A. Enhanced Topical Delivery of Cyclosporin-A Using PLGA Nanoparticles as Carrier. Curr. Nanosci. 2011, 7. [Google Scholar] [CrossRef]
- Lapteva, M.; Santer, V.; Mondon, K.; Patmanidis, I.; Chiriano, G.; Scapozza, L.; Gurny, R.; Moller, M.; Kalia, Y.N. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J. Control. Release 2014, 196, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.; Kohn, J. Formulation Strategy for the Delivery of Cyclosporine A: Comparison of Two Polymeric Nanospheres. Sci. Rep. 2015, 5, 13065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musa, S.H.; Basri, M.; Fard Masoumi, H.R.; Shamsudin, N.; Salim, N. Enhancement of physicochemical properties of nanocolloidal carrier loaded with cyclosporine for topical treatment of psoriasis: In vitro diffusion and in vivo hydrating action. Int. J. Nanomed. 2017, 12, 2427–2441. [Google Scholar] [CrossRef] [Green Version]
- Badihi, A.; Frusic-Zlotkin, M.; Soroka, Y.; Benhamron, S.; Tzur, T.; Nassar, T.; Benita, S. Topical nano-encapsulated cyclosporine formulation for atopic dermatitis treatment. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102140. [Google Scholar] [CrossRef]
- Carreras, J.J.; Tapia-Ramirez, W.E.; Sala, A.; Guillot, A.J.; Garrigues, T.M.; Melero, A. Ultraflexible lipid vesicles allow topical absorption of cyclosporin A. Drug Deliv. Transl. Res. 2020, 10, 486–497. [Google Scholar] [CrossRef]
- Essaghraoui, A.; Belfkira, A.; Hamdaoui, B.; Nunes, C.; Lima, S.A.C.; Reis, S. Improved Dermal Delivery of Cyclosporine A Loaded in Solid Lipid Nanoparticles. Nanomaterials 2019, 9, 1204. [Google Scholar] [CrossRef] [Green Version]
- IOI OLEOCHEMICAL. Softisan®649—A Vegan Alternative to Lanolin; IOI Oleo GmbH: Hamburg, Germany, 2018. [Google Scholar]
- Vaananen, A.; Hannuksela, M. Softisan—A new vehicle for patch testing. Contact Derm. 1986, 14, 215–216. [Google Scholar] [CrossRef]
- Osborne, D.W. Phase Behavior Characterization of Ointments Containing Lanolin or a Lanolin Substitute. Drug Dev. Ind. Pharm. 2008, 19, 1283–1302. [Google Scholar] [CrossRef]
- Haisma, E.M.; Goblyos, A.; Ravensbergen, B.; Adriaans, A.E.; Cordfunke, R.A.; Schrumpf, J.; Limpens, R.W.; Schimmel, K.J.; den Hartigh, J.; Hiemstra, P.S.; et al. Antimicrobial Peptide P60.4Ac-Containing Creams and Gel for Eradication of Methicillin-Resistant Staphylococcus aureus from Cultured Skin and Airway Epithelial Surfaces. Antimicrob. Agents Chemother. 2016, 60, 4063–4072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faqi, A.S.; Bell, S.J.; Gill, S.; Colagiovanni, D.B. An intranasal irritation assessment of antibacterial ointment alone or in combination with mupirocin versus Bactroban Nasal in rabbits. Regul. Toxicol. Pharmacol. 2009, 55, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.T.; Cade, D.; Benameur, H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv. Drug Deliv. Rev. 2008, 60, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Matl, F.D.; Obermeier, A.; Repmann, S.; Friess, W.; Stemberger, A.; Kuehn, K.D. New anti-infective coatings of medical implants. Antimicrob. Agents Chemother. 2008, 52, 1957–1963. [Google Scholar] [CrossRef] [Green Version]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Al-Saedi, Z.H.; Alzhrani, R.M.; Boddu, S.H. Formulation and In Vitro Evaluation of Cyclosporine-A Inserts Prepared Using Hydroxypropyl Methylcellulose for Treating Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2016, 32, 451–462. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of bis-diglyceryl polyacyladipate-2 and bis-diglyceryl polyacyladipate-1 as used in cosmetics. Int. J. Toxicol. 2013, 32, 56S–64S. [Google Scholar] [CrossRef]
- Ridolfi, D.M.; Marcato, P.D.; Machado, D.; Silva, R.A.; Justo, G.Z.; Durán, N. In vitro cytotoxicity assays of solid lipid nanoparticles in epithelial and dermal cells. J. Phys. Conf. Ser. 2011, 304, 012032. [Google Scholar] [CrossRef]
- Vranic, S.; Boggetto, N.; Contremoulins, V.; Mornet, S.; Reinhardt, N.; Marano, F.; Baeza-Squiban, A.; Boland, S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part. Fibre Toxicol. 2013, 10, 2. [Google Scholar] [CrossRef]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Damodar, G.; Sughir, A. Oleogel: A promising base for transdermal formulations. Asian J. Pharm. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Herbig, M.E.; Houdek, P.; Gorissen, S.; Zorn-Kruppa, M.; Wladykowski, E.; Volksdorf, T.; Grzybowski, S.; Kolios, G.; Willers, C.; Mallwitz, H.; et al. A custom tailored model to investigate skin penetration in porcine skin and its comparison with human skin. Eur. J. Pharm. Biopharm. 2015, 95, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.P.; Scott, R.C. Pig ear skin as an in-vitro model for human skin permeability. J. Pharm. Pharmacol. 1992, 44, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, M.; Kawaguchi, K.; Ohsuga, Y.; Ikari, Y.; Yanagawa, A.; Mizushima, Y. Cyclosporin ointment for psoriasis and atopic dermatitis. Lancet 1992, 339, 1120. [Google Scholar] [CrossRef]
- Alvarez-Figueroa, M.J.; Abarca-Riquelme, J.M.; González-Aramundiz, J.V. Influence of protamine shell on nanoemulsions as a carrier for cyclosporine-A skin delivery. Pharm. Dev. Technol. 2019, 24, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.B.; Arntjen, A.; Keck, C.M.; Müller, R.H. Amorphous cyclosporin A nanoparticles for enhanced dermal bioavailability. Int. J. Pharm. 2016, 498, 217–224. [Google Scholar] [CrossRef]
- Mondon, K.; Zeisser-Labouebe, M.; Gurny, R.; Moller, M. Novel cyclosporin A formulations using MPEG-hexyl-substituted polylactide micelles: A suitability study. Eur. J. Pharm. Biopharm. 2011, 77, 56–65. [Google Scholar] [CrossRef]








| Size (nm) | PDI | ζ-Potential (mV) | EE (%) | DL (%) | |
|---|---|---|---|---|---|
| SLN | 200 ± 4 | 0.12 ± 0.03 | −15 ± 4 | - | - |
| CsA-SLN | 216 ± 5 | 0.11 ± 0.02 | −22 ± 2 | 88 ± 3 | 6.6 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.I.; Barbosa, A.I.; Costa Lima, S.A.; Costa, P.; Torres, T.; Reis, S. Freeze-Dried Softisan® 649-Based Lipid Nanoparticles for Enhanced Skin Delivery of Cyclosporine A. Nanomaterials 2020, 10, 986. https://doi.org/10.3390/nano10050986
Silva MI, Barbosa AI, Costa Lima SA, Costa P, Torres T, Reis S. Freeze-Dried Softisan® 649-Based Lipid Nanoparticles for Enhanced Skin Delivery of Cyclosporine A. Nanomaterials. 2020; 10(5):986. https://doi.org/10.3390/nano10050986
Chicago/Turabian StyleSilva, Maria Inês, Ana Isabel Barbosa, Sofia A. Costa Lima, Paulo Costa, Tiago Torres, and Salette Reis. 2020. "Freeze-Dried Softisan® 649-Based Lipid Nanoparticles for Enhanced Skin Delivery of Cyclosporine A" Nanomaterials 10, no. 5: 986. https://doi.org/10.3390/nano10050986