Nano MnO2 Radially Grown on Lignin-Based Carbon Fiber by One-Step Solution Reaction for Supercapacitors with High Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
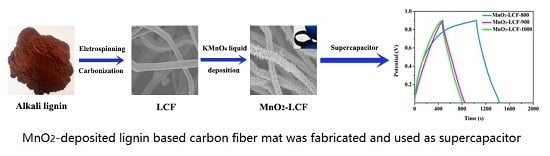
2.2. Preparation of LCFs
2.3. Preparation of MnO2-deposited LCF (MnO2-LCF) Mats
2.4. Characterization of LCF and MnO2-LCF
2.5. Electrochemical Performance of MnO2-LCF
3. Results and Discussion
3.1. Effect of Carbonization Temperature on the Morphology and Structure of the LCF Mats
3.2. Morphology and Structure of MnO2-LCF
3.3. Electrochemical Performance of MnO2-LCF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- In Proceedings of theZhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon Materials for Chemical Capacitive Energy Storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Li, F.; Wang, Z.; Hu, N.; Wen, Z.; Liu, Q. Merging of Kirkendall Growth and Ostwald Ripening: CuO@MnO2 Core-shell Architectures for Asymmetric Supercapacitors. Sci. Rep. 2014, 4, 4518. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Aradilla, D.; Bidan, G.; Gentile, P.; Schubert, T.J.S.; Wimberg, J.; Sadki, S.; Gomez-Romero, P. 3D hierarchical assembly of ultrathin MnO2 nanoflakes on silicon nanowires for high performance micro-supercapacitors in Li- doped ionic liquid. Sci. Rep.-UK. 2015, 5, 9771. [Google Scholar] [CrossRef]
- Ye, Z.; Jiao, Y.; Meng, L.; Wang, B.; Peng, H. Carbon nanomaterials for flexible lithium ion batteries. Carbon 2017, 124, 79–88. [Google Scholar]
- Yang, X.; Zhang, L.; Zhang, F.; Zhang, T.; Huang, Y.; Chen, Y. Chen A high-performance all-solid-state supercapacitor with graphene-doped carbon material electrodes and a graphene oxide-doped ion gel electrolyte. Carbon 2014, 72, 381–386. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Young, M.J.; Schnabel, H.; Holder, A.M.; George, S.M.; Musgrave, C.B. Band Diagram and Rate Analysis of Thin Film Spinel LiMn2O4 Formed by Electrochemical Conversion of ALD-Grown MnO. Adv. Funct. Mater. 2016, 26, 7895–7907. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2, Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Chen, I.L.; Chen, T.Y.; Wei, Y.C.; Hu, C.C.; Lin, T.L. Capacitive performance enhancements of RuO2 nanocrystals through manipulation of preferential orientation growth originated from the synergy of Pluronic F127 trapping and annealing. Nanoscale 2014, 6, 2861–2871. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kang, F. A high-performance asymmetric supercapacitor based on carbon and carbon–MnO2 nanofiber electrodes. Carbon 2013, 61, 190–199. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.J.; Dong, J.L.; Song, J.; Park, J.K. Directly Grown Co3O4 Nanowire Arrays on Ni-foam: Structural Effect of Carbon-free and Binder-free Cathodes for Lithium Oxygen Batteries. J. Mater. Chem. A 2014, 2, 11891–11898. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.L.; Ji, H.; Li, Y.; Ruoff, R.S. Nanoporous Ni(OH)2 Thin Film on 3D Ultrathin-Graphite Foam for Asymmetric Supercapacitor. Acs Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Sun, Y.; Lei, F.; Liang, L.; Liu, J.; Bi, W.; Pan, B.; Xie, Y. Ultrahigh energy density realized by a single-layer β-Co(OH)2 all-solid-state asymmetric supercapacitor. Chem. Int. Edit. 2015, 46, 12789–12793. [Google Scholar]
- Zhao, L.; Yu, J.; Li, W.; Wang, S.; Dai, C.; Wu, J.; Bai, X.; Zhi, C. Honeycomb porous MnO2 nanofibers assembled from radially grown nanosheets for aqueous supercapacitors with high working voltage and energy density. Nano Energy 2014, 4, 39–48. [Google Scholar] [CrossRef]
- Pang, S.C.; Anderson, M.A.; Chapman, T.W. Novel Electrode Materials for Thin-Film Ultracapacitors: Comparison of Electrochemical Properties of Sol-Gel-Derived and Electrodeposited Manganese Dioxide. Electrochem. Soc. 2000, 147, 444–450. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid. State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.A.; An, N.; Yang, Y.Y.; Li, Z.M.; Wu, H.Y. Facile Synthesis of MnO2/CNTs Composite for Supercapacitor Electrodes with Long Cycle Stability. J. Phys. Chem. C 2014, 118, 22865–22872. [Google Scholar] [CrossRef]
- Dai, K.; Lu, L.; Liang, C.; Dai, J.; Liu, Q.; Zhang, Y.; Zhu, G.; Liu, Z. In situ assembly of MnO2 nanowires/graphene oxide nanosheets composite with high specific capacitance. Electrochim. Acta 2014, 116, 111–117. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Yu, H.; Wu, H.; Wang, J.; Liu, A. MnO2 nanowires-decorated carbon fiber cloth as electrodes for aqueous asymmetric supercapacitor. Funct. Mater. Lett. 2018, 11, 1850034. [Google Scholar] [CrossRef]
- Youe, W.J.; Kim, S.J.; Lee, S.M.; Chun, S.J.; Kang, J.; Kim, Y.S. MnO2-deposited lignin-based carbon nanofiber mats for application as electrodes in symmetric pseudocapacitors. Int. J. Biol. Macromol. 2018, 112, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Y.; Luo, Y.; Chu, P.K. Highly porous honeycomb manganese oxide@carbon fibers core–shell nanocables for flexible supercapacitors. Nano Energy 2015, 13, 47–57. [Google Scholar] [CrossRef]
- Karacan, I.; Erzurumluoğlu, L. The effect of carbonization temperature on the structure and properties of carbon fibers prepared from poly (m-phenylene isophthalamide) precursor. Fiber. Polym. 2015, 16, 1629–1645. [Google Scholar] [CrossRef]
- Kil, H.S.; Oh, K.; Kim, Y.J.; Ko, S.; Jeon, Y.P.; Joh, I.H.; Kim, Y.K.; Lee, S. Structural evolution of pitch fibers during low temperature carbonization. J. Anal. Appl Pyrol. 2018, 136, 153–159. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, H.G.; Kang, S.J.; Park, S.J.; An, K.H.; Kim, B.J. Effects of pore structures on electrochemical behaviors of polyacrylonitrile (PAN)-based activated carbon nanofibers. J. Ind. Eng. Chem. 2015, 21, 736–740. [Google Scholar] [CrossRef]
- Jishi, R.A.; Elman, B.S.; Dresselhaus, G. Lattice dynamical model for graphite. Phys. Rev. B 1982, 20, 127. [Google Scholar]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 2002, 2, 557–563. [Google Scholar] [CrossRef]
- Woodhead, A.L.; de Souza, M.L.; Church, J.S. An investigation into the surface heterogeneity of nitric acid oxidized carbon fiber. Appl. Surf. Sci. 2017, 401, 79–88. [Google Scholar] [CrossRef]
- Chatterjee, S.; Jones, E.B.; Clingenpeel, A.C.; McKenna, A.M.; Rios, O.; McNutt, N.W.; Keffer, D.J.; Johs, A. Conversion of Lignin Precursors to Carbon Fibers with Nanoscale Graphitic Domains. ACS Sustain. Chem. Eng. 2014, 2, 2002–2010. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Gudavalli, G.S.; Turner, J.N.; Dhakal, T.P. Pulse electrodeposited manganese oxide on carbon fibers as electrodes for high capacity supercapacitors. Nanotechnology 2019, 30, 455701. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Yang, K.S.; Yang, D.J. Electrochemical behavior of activated carbon nanofiber-vanadium pentoxide composites for double-layer capacitors. Electrochim. Acta 2013, 109, 859–865. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Feizbakhshan, M.; Liu, C.; Hong, S.; Yang, P.; Zhou, X. Preparation of lignin-based porous carbon with hierarchical oxygen-enriched structure for high-performance supercapacitors. J. Colloid. Interf. Sci. 2019, 540, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, B.; Du, H.; Kang, F.; Zeng, Y. Electrochemical properties of nanosized hydrous manganese dioxide synthesized by a self-reacting microemulsion method. J. Power. Sources 2008, 180, 664–670. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, X.H.; Xiao, X.; Zhai, T.; Dai, J.; Zhang, F.; Hu, B.; Wang, X.; Gong, L.; Chen, J. Flexible Solid-State Supercapacitors Based on Carbon Nanoparticles/MnO2 Nanorods Hybrid Structure. ACS Nano 2012, 6, 656–661. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, P.; Chen, L. Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures. Appl. Catal. B -Environ. 2016, 189, 210–218. [Google Scholar] [CrossRef]
- Aschauer, U.; Vonrüti, N.; Spaldin, N.A. Effect of epitaxial strain on cation and anion vacancy formation in MnO. Phys. Rev. B 2015, 92, 054103. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, A.; Inoue, S.; Akamaru, S.; Hara, M.; Watanabe, K.; Abe, T. Phase transition and electrochemical capacitance of mechanically treated manganese oxides. J. Alloy. Compd. 2006, 414, 137–141. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, J.P. Raman spectra of birnessite manganese dioxides. Solid State Ionics 2003, 159, 345–356. [Google Scholar] [CrossRef]
- Ma, X.; Kolla, P.; Zhao, Y.; Smirnova, A.L.; Fong, H. Electrospun lignin-derived carbon nanofiber mats surface-decorated with MnO2 nanowhiskers as binder-free supercapacitor electrodes with high performance. J. Power Sources 2016, 325, 541–548. [Google Scholar] [CrossRef]
- Li, L.Z.; Wei, T.; Wang, W.; Zhao, X.S. Manganese oxide–carbon composite as supercapacitor electrode materials. Micropor. Mesopor. Mater. 2009, 123, 260–267. [Google Scholar]
- Zhou, H.; Han, G.; Xiao, Y.; Chang, Y.; Zhai, H.J. Facile preparation of polypyrrole/grapheme oxide nanocomposites with large areal capacitance using electrochemical codeposition for supercapacitors. J. Power Sources 2014, 263, 259–267. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, X.; Liu, Y.; Cai, Q.; Zhang, J. One-pot hydrothermal synthesis of Mn3O4/graphene nanocomposite for supercapacitors. Mater. Lett. 2013, 95, 153–156. [Google Scholar] [CrossRef]






| Sample | SBET (m2∙g−1) | Vmic (cm3∙g−1) | Vt (cm3∙g−1) | Vmic/Vt |
|---|---|---|---|---|
| MnO2-LCF-800 | 273.70 | 0.03 | 0.09 | 33.33 |
| MnO2-LCF-900 | 210.91 | 0.04 | 0.07 | 57.14 |
| MnO2-LCF-1000 | 385.16 | 0.08 | 0.12 | 66.67 |
| Sample | Atomic Percentage of C (%) | Atomic Percentage of Mn (%) | Atomic Percentage of O (%) |
|---|---|---|---|
| MnO2-LCF-800 | 54.54 | 19.30 | 26.16 |
| MnO2-LCF-900 | 51.61 | 19.52 | 25.62 |
| MnO2-LCF-1000 | 54.54 | 19.84 | 25.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Ma, H.; Zhang, Q.; Li, M.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Nano MnO2 Radially Grown on Lignin-Based Carbon Fiber by One-Step Solution Reaction for Supercapacitors with High Performance. Nanomaterials 2020, 10, 594. https://doi.org/10.3390/nano10030594
Guo C, Ma H, Zhang Q, Li M, Jiang H, Chen C, Wang S, Min D. Nano MnO2 Radially Grown on Lignin-Based Carbon Fiber by One-Step Solution Reaction for Supercapacitors with High Performance. Nanomaterials. 2020; 10(3):594. https://doi.org/10.3390/nano10030594
Chicago/Turabian StyleGuo, Chenyan, Haitong Ma, Qingtong Zhang, Mingfu Li, Hongrui Jiang, Changzhou Chen, Shuangfei Wang, and Douyong Min. 2020. "Nano MnO2 Radially Grown on Lignin-Based Carbon Fiber by One-Step Solution Reaction for Supercapacitors with High Performance" Nanomaterials 10, no. 3: 594. https://doi.org/10.3390/nano10030594