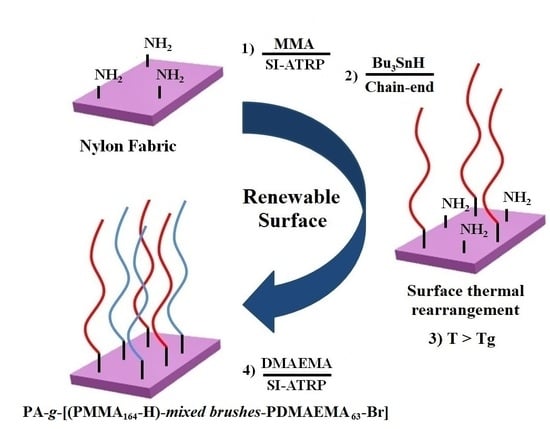
Renewable Fabric Surface-Initiated ATRP Polymerizations: Towards Mixed Polymer Brushes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of PA Knitted Fabric
2.2.1. PA Modification with ATRP Initiator: PA-Br
2.2.2. Grafting of Poly(Methyl Methacrylate) from PA Surface via SI-ATRP: PA-g-PMMA164-Br
2.2.3. Deactivation of PA-g-PMMA164-Br Chains Ends with Tributyltin Hydride (Bu3SnH): PA-g-PMMA164-H
2.2.4. Rearrangement of PA-g-PMMA164-H
2.2.5. Modification PA-g-PMMA164-H with an ATRP Initiator: PA-g-[(PMMA164-H), Br]
2.2.6. Synthesis of Mixed-Polymer Brushes: PA-g-[(PMMA164-H)-mixed brushes-PDMAEMA63-Br]
2.2.7. Loading Gold Precursor HAuCl4 onto PA-g-[(PMMA164-H)-mixed brushes-PDMAEMA63-Br]: AuNPs@ PA-g-[(PMMA164-H)-mixed brushes-PDMAEMA63-Br]
2.3. Characterization of Surface Modified PA Knitted Fabric
2.3.1. Nuclear Magnetic Resonance Characterization
2.3.2. Gel Permeation Chromatography Characterization
2.3.3. Fourier Transform Infrared Spectroscopy Characterization
2.3.4. Elemental Analysis
2.3.5. Scanning Electron Microscopy–Energy-Dispersive Spectroscopy Characterization, SEM-EDS
2.3.6. Contact Angle Characterization
2.3.7. Measurement of Bacteriostatic Properties
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Barbey, R.; Lavanant, L.; Paripovic, D.; Schuwer, N.; Sugnaux, C.; Tugulu, S.; Klok, H.-A. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chem. Rev. 2009, 109, 5435–5527. [Google Scholar] [CrossRef]
- Hui, C.M.; Pietrasik, J.; Schmitt, M.; Mahoney, C.; Choi, J.; Bockstaller, M.R.; Matyjaszewski, K. Surface-Initiated Polymerization as an Enabling Tool for Multifunctional (Nano-)Engineered Hybrid Materials. Chem. Mater. 2013, 26, 745–762. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Fritz, G.; Schadler, V.; Willenbacher, N.; Wagner, N.J. Electrosteric Stabilization of Colloidal Dispersions. Langmuir 2002, 18, 6381–6390. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietraisk, J.; Kusumo, A. Grafting from Surfaces for “Everyone”: ARGET ATRP in thePresence of Air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sun, X.; Wu, C.; Hu, J.; Huang, X. Construction of catechol-containing semi-fluorinated asymmetric polymer brush via successive RAFT polymerization and ATRP. Polym. Chem. 2017, 8, 7499–7506. [Google Scholar] [CrossRef]
- Krysiak, E.; Janasz, L.; Dupont, B.G.R.; Wypych-Puszkarz, A.; Matyjaszewski, K.; Ulanski, J. Poly(2-hydroxyethyl methacrylate) brushes synthesized by atom transfer radical polymerization from gold surface as a gate insulator in organic thin-film transistors. Thin Solid Films 2019, 669, 133–140. [Google Scholar] [CrossRef]
- Hadjesfandiari, N.; Yu, K.; Mei, Y.; Kizhakkedathu, J.N. Polymer brush-based approaches for the development of infection-resistant surfaces. J. Mater. Chem. B 2014, 2, 4968–4978. [Google Scholar] [CrossRef]
- Nath, N.; Chilkoti, A. Creating "Smart" Surfaces Using Stimuli Responsive Polymers. Adv. Mater. 2002, 14, 1243–1247. [Google Scholar] [CrossRef]
- Panzarasa, G.; Soliveri, G.; Ardizzone, S.; Sparnacci, K. Photocatalytic Lithography: An Innovative Approach to Obtain Patterned pH-responsive Polymer Brushes. Mater. Today Proc. 2015, 2, 4183–4189. [Google Scholar] [CrossRef]
- Calabrese, D.R.; Ditter, D.; Liedel, C.; Blumfield, A.; Zentel, R.; Ober, C.K. Design, Synthesis, and Use of Y-Shaped ATRP/NMP Surface Tethered Initiator. ACS Macro Lett. 2015, 4, 606–610. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Liu, Y.; Klep, V.; Zdyrko, B.; Luzinov, I. Polymer Grafting via ATRP Initiated from Macroinitiator Synthesized on Surface. Langmuir 2004, 20, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O. Polymer brushes here, there, and everywhere: Recent advances in their practical applications and emerging opportunities in multiple research fields. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3225–3258. [Google Scholar] [CrossRef]
- Tsujii, Y.; Ohno, K.; Yamamoto, S.; Goto, A.; Fukuda, T. Structure and Properties of High-Density Polymer Brushes Prepared by Surface-Initiated Living Radical Polymerization. Adv. Polym. Sci. 2006, 197, 1–45. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; et al. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar]
- Chiefari, J.; Chong, Y.K.B.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Tomlinson, M.R.; Efimenko, K.; Genzer, J. Study of kinetics and macroinitiator efficiency in surface-initiated atom-transfer radical polymerization. Macromolecules 2006, 39, 9049–9056. [Google Scholar] [CrossRef]
- Keddie, D.J.; Moad, G.; Rizzardo, E.; Thang, S.H. RAFT Agent Design and Synthesis. Macromolecules 2012, 45, 5321–5342. [Google Scholar] [CrossRef]
- Allen, M.J.; Wangkanont, K.; Raines, R.T.; Kiessling, L.L. ROMP from ROMP: A New Approach to Graft Copolymer Synthesis. Macromolecules 2009, 42, 4023–4027. [Google Scholar] [CrossRef] [Green Version]
- Henze, M.; Mädge, D.; Prucker, O.; Rühe, J. “Grafting Through”: Mechanistic Aspects of Radical Polymerization Reactions with Surface-Attached Monomers. Macromolecules 2014, 47, 2929–2937. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Xiao, A.; Yu, H. The synthesis of modified polyethylene via coordination polymerization followed by ATRP, RAFT, NMRP or ROP. Prog. Polym. Sci. 2010, 35, 1195–1216. [Google Scholar] [CrossRef]
- Roling, O.; De Bruycker, K.; Vonhören, B.; Stricker, L.; Körsgen, M.; Arlinghaus, H.F.; Ravoo, B.J.; Du Prez, F.E. Herstellung mikrostrukturierter Polymerbürsten auf wiederbeschreibbaren Oberflächen durch Triazolindion-Click-Chemie. Angew. Chem. 2015, 127, 13319–13323. [Google Scholar] [CrossRef]
- Roling, O.; De Bruycker, K.; Vonhoren, B.; Stricker, L.; Korsgen, M.; Arlinghaus, H.F.; Ravoo, B.J.; Du Prez, F.E. Rewritable Polymer Brush Micropatterns Grafted by Triazolinedione Click Chemistry. Angew Chem. Int. Ed. Engl. 2015, 54, 13126–13129. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, B.; Wang, X.; Yu, B.; Pei, X.; Huck, W.T.; Zhou, F. Bio-Inspired Renewable Surface-Initiated Polymerization from Permanently Embedded Initiators. Angew Chem. Int. Ed. Engl. 2016, 55, 4260–4264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhong, G.; Horton, J.M.; Jin, N.; Zhu, L.; Zhao, B. Evolution of Phase Morphology of Mixed Poly(tert-butyl acrylate)/Polystyrene Brushes Grafted on Silica Particles with the Change of Chain Length Disparity. Macromolecules 2010, 43, 5387–5395. [Google Scholar] [CrossRef]
- Bao, C.; Tang, S.; Horton, J.M.; Jiang, X.; Tang, P.; Qiu, F.; Zhu, L.; Zhao, B. Effect of Overall Grafting Density on Microphase Separation of Mixed Homopolymer Brushes Synthesized from Y-Initiator-Functionalized Silica Particles. Macromolecules 2012, 45, 8027–8036. [Google Scholar] [CrossRef]
- Li, W.; Bao, C.; Wright, R.A.E.; Zhao, B. Synthesis of mixed poly(ε-caprolactone)/polystyrene brushes from Y-initiator-functionalized silica particles by surface-initiated ring-opening polymerization and nitroxide-mediated radical polymerization. RSC Adv. 2014, 4, 18772–18781. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, L. Mixed Polymer Brush-Grafted Particles: A New Class of Environmentally Responsive Nanostructured Materials. Macromolecules 2009, 42, 9369–9383. [Google Scholar] [CrossRef]
- Draper, J.; Luzinov, I.; Minko, S.; Tokarev, I.; Stamm, M. Mixed Polymer Brushes by Sequential Polymer Addition: Anchoring Layer Effect. Langmuir 2004, 20, 4064–4075. [Google Scholar] [CrossRef]
- Zhao, B. A combinatorial approach to study solvent-induced self-assembly of mixed poly(methyl methacrylate)/polystyrene brushes on planar silica substrates: Effect of relative grafting density. Langmuir 2004, 20, 11748–11755. [Google Scholar] [CrossRef]
- Sidorenko, A.; Minko, S.; Schenk-Meuser, K.; Duschner, H.; Stamm, M. Switching of Polymer Brushes. Langmuir 1999, 15, 8349–8355. [Google Scholar] [CrossRef]
- Ionov, L.; Sidorenko, A.; Stamm, M.; Minko, S.; Zdyrko, B.; Klep, V.; Luzinov, I. Gradient Mixed Brushes: “Grafting To” Approach. Macromolecules 2004, 37, 7421–7423. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of Monodisperse Silica Particles Coated with Well-Defined, High-Density Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2005, 38, 2137–2142. [Google Scholar] [CrossRef]
- Martinez, A.P.; Carrillo, J.-M.Y.; Dobrynin, A.V.; Adamson, D.H. Distribution of Chains in Polymer Brushes Produced by a “Grafting From” Mechanism. Macromolecules 2016, 49, 547–553. [Google Scholar] [CrossRef]
- Huang, Y.; Yong, P.; Chen, Y.; Gao, Y.; Xu, W.; Lv, Y.; Yang, L.; Reis, R.L.; Pirraco, R.; Wang, K. Micellization and Gelatinization in Aqueous Media of pH- and Thermo-Responsive Amphiphilic ABC (PMMA82-b-PDMAEMA150 -b-PNIPAM65) Triblock Copolymer Synthesized by Consecutive RAFT Polymerization. RSC Advances 2017, 7, 28711–28722. [Google Scholar] [CrossRef] [Green Version]
- Kassaee, M.Z.; Motamedi, E.; Majdi, M. Magnetic Fe3O4-graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite. Chem. Eng. J. 2011, 172, 540–549. [Google Scholar] [CrossRef]
- Bormashenko, E. General equation describing wetting of rough surfaces. J. Colloid. Interface Sci. 2011, 360, 317–319. [Google Scholar] [CrossRef]
- Giljean, S.; Bigerelle, M.; Anselme, K.; Haidara, H. New insights on contact angle/roughness dependence on high surface energy materials. Appl. Surface Sci. 2011, 257, 9631–9638. [Google Scholar] [CrossRef]
- Cansoy, C.E.; Erbil, H.Y.; Akar, O.; Akin, T. Effect of pattern size and geometry on the use of Cassie–Baxter equation for superhydrophobic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 116–124. [Google Scholar] [CrossRef]
- Buchanan, D.R.; Walters, J.P. Glass-Transition Temperatures of Polyamide Textile Fibers:Part I: The Effects of Molecular Structure, Water, Fiber Structure, and Experimental Technique. Text. Res. J. 1977, 47, 398–406. [Google Scholar] [CrossRef]
- Coessens, V.; Matyjaszewski, K. Dehalogenation of polymers prepared by atom transfer radical polymerization. Macromol. Rapid Commun. 1999, 20, 66–70. [Google Scholar] [CrossRef]
- Gianotti, V.; Antonioli, D.; Sparnacci, K.; Laus, M.; Giammaria, T.J.; Ferrarese Lupi, F.; Seguini, G.; Perego, M. On the Thermal Stability of PS-b-PMMA Block and P(S-r-MMA) Random Copolymers for Nanopatterning Applications. Macromolecules 2013, 46, 8224–8234. [Google Scholar] [CrossRef]
- Pietrasik, J.; Sumerlin, B.S.; Lee, R.Y.; Matyjaszewski, K. Solution Behavior of Temperature-Responsive Molecular Brushes Prepared by ATRP. Macromol. Chem. Phys. 2007, 208, 30–36. [Google Scholar] [CrossRef]
- Santos, F.G.; Bonkovoski, L.C.; Garcia, F.P.; Cellet, T.S.; Witt, M.A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Antibacterial Performance of a PCL-PDMAEMA Blend Nanofiber-Based Scaffold Enhanced with Immobilized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 9304–9314. [Google Scholar] [CrossRef] [PubMed]





| Entry | Sample | Mtheora | Mnb | Mw/Mn |
|---|---|---|---|---|
| 1 | PA-g-PMMA164-Br | 16,400 | 17,400 | 1.11 |
| 2 | PA-g-[(PMMA164-H)-mixed brushes-PDMAEMA63-Br] | 9400 | 11,600 | 1.13 |
| 3 | PA-g-PS66-Br | 7000 | 7500 | 1.18 |
| 4 | PA-g-[(PS66-TEMPO)-mixed brushes-PDMAEMA119-Br] | 20,600 | 17,900 | 1.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, W.; Russo, A.; Zhang, Y.; Chapelat, J.; Pietrasik, J. Renewable Fabric Surface-Initiated ATRP Polymerizations: Towards Mixed Polymer Brushes. Nanomaterials 2020, 10, 536. https://doi.org/10.3390/nano10030536
Raj W, Russo A, Zhang Y, Chapelat J, Pietrasik J. Renewable Fabric Surface-Initiated ATRP Polymerizations: Towards Mixed Polymer Brushes. Nanomaterials. 2020; 10(3):536. https://doi.org/10.3390/nano10030536
Chicago/Turabian StyleRaj, Wojciech, Alessandro Russo, Yaoming Zhang, Julien Chapelat, and Joanna Pietrasik. 2020. "Renewable Fabric Surface-Initiated ATRP Polymerizations: Towards Mixed Polymer Brushes" Nanomaterials 10, no. 3: 536. https://doi.org/10.3390/nano10030536