Gas Sensing with Solar Cells: The Case of NH3 Detection through Nanocarbon/Silicon Hybrid Heterojunctions
Abstract
:1. Introduction
2. Materials and Methods
2.1. PV Cell Preparation
2.2. Gas Exposure
2.3. Electrical Measurements
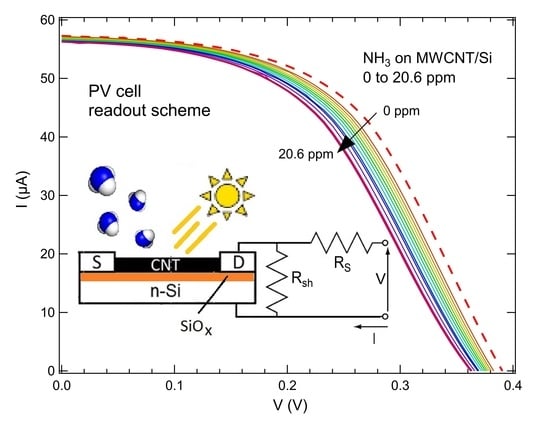
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gad, A.; Hoffmann, M.W.G.; Prades, J.D.; Hernandez-Ramirez, F.; Fiz, R.; Shen, H.; Mathur, S.; Waag, A. Self-Powered Solar Diode Gas Sensors. In Proceedings of the 2014 International Conference on Solid State Devices and Materials, Tsukuba, Japan, 8–11 September 2014; Volume 572, pp. 572–573. [Google Scholar]
- Fang, H.-H.; Adjokatse, S.; Wei, H.; Yang, J.; Blake, G.R.; Huang, J.; Even, J.; Loi, M.A. Ultrahigh sensitivity of methylammonium lead tribromide perovskite single crystals to environmental gases. Sci. Adv. 2016, 2, e1600534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; Park, J.; Kim, B.; Na, K.; Cho, I.; Rho, J.; Yang, D.; Lee, J.Y.; Park, I. A self-powered gas sensor based on a photovoltaic cell and a colorimetric film with hierarchical micro/nano-structures. ACS Appl. Mater. Interfaces 2020, 12, 39024–39032. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Na, K.; Lee, J.Y.; Park, I. Development of NO2 Gas Sensor Using Colorimetric Film and Organic Solar Cell in Self-powered System. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS, Vienna, Austria, 15–19 July 2018; pp. 466–467. [Google Scholar] [CrossRef]
- Vashpanov, Y.; Son, J.Y.; Heo, G.; Kwack, K.D. Photovoltaic intelligent gas sensors for the detection of acetone concentration over a wide range of measurement for biomedical applications and tasks of public safety. IOP Conf. Ser. Mater. Sci. Eng. 2020, 715, 012094. [Google Scholar] [CrossRef]
- Tune, D.D.; Mallik, N.; Fornasier, H.; Flavel, B.S. Breakthrough Carbon Nanotube-Silicon Heterojunction Solar Cells. Adv. Energy Mater. 2019, 10, 1903261. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Batmunkh, M.; Shearer, C.; Shapter, J.G. Use of Carbon Nanotubes (CNTs) in Third-Generation Solar Cells. In Emerging Photovoltaic Materials: Silicon and Beyon; Kurinec, S.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 551–609. [Google Scholar]
- Tune, D.D.; Flavel, B.S. Advances in Carbon Nanotube–Silicon Heterojunction Solar Cells. Adv. Energy Mater. 2018, 8, 1703241. [Google Scholar] [CrossRef]
- Yu, L.; Grace, T.; Batmunkh, M.; Dadkhah, M.; Shearer, C.; Shapter, J. Insights into chemical doping to engineer the carbon nanotube/silicon photovoltaic heterojunction interface. J. Mater. Chem. A 2017, 5, 24247–24256. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lv, Z.; Zhu, H. Carbon/Silicon Heterojunction Solar Cells: State of the Art and Prospects. Adv. Mater. 2015, 27, 6549–6574. [Google Scholar] [CrossRef]
- Castrucci, P. Carbon Nanotube/Silicon Hybrid Heterojunctions for Photovoltaic Devices. Adv. Nano Res. 2014, 2, 23–56. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huang, J.S.; Nejati, S.; McMillon, L.; Huang, S.; Osuji, C.O.; Hazari, N.; Taylor, A.D. Role of HF in Oxygen Removal from Carbon Nanotubes: Implications for High Performance Carbon Electronics. Nano Lett. 2014, 14, 6179–6184. [Google Scholar] [CrossRef]
- Tune, D.D.; Flavel, B.S.; Krupke, R.; Shapter, J.G. Carbon Nanotube-Silicon Solar Cells. Adv. Energy Mater. 2012, 2, 1043–1055. [Google Scholar] [CrossRef]
- Chernov, A.I.; Eremina, V.A.; Shook, J.; Collins, A.; Walker, P.; Fedotov, P.V.; Zakhidov, A.A.; Obraztsova, E.D. Field Effect Transistor Based on Solely Semiconducting Single-Walled Carbon Nanotubes for the Detection of 2-Chlorophenol. Phys. Stat. Sol. B 2018, 255, 1700139. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, G.H.; Wang, Y.; Wang, Y.Y.; Li, T.; Zhang, T.; Qin, S.J. A photovoltaic self-powered gas sensor based on a single-walled carbon nanotube/Si heterojunction. Nanoscale 2017, 9, 18579. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, F.; Pintossi, C.; Drera, G.; Pagliara, S.; Lanti, G.; Castrucci, P.; De Crescenzi, M.; Sangaletti, L. A cross-functional nanostructured platform based on carbon nanotube-Si hybrid junctions: Where photon harvesting meets gas sensing. Sci. Rep. 2017, 7, 44413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Wei, J.Q.; Jia, Y.; He, S.Q.; Sun, H.H.; Zhu, H.W.; Wang, K.L.; Wu, D.H. The influence of gas absorption on the efficiency of carbon nanotube/Si solar cells. Appl. Phys. Lett. 2013, 102, 143105. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Fan, L.; Li, Z.; Bai, X.; Mulligan, S.; Jia, Y.; Wang, K.; Wei, J.; Cao, A.; Wu, D.; et al. Hybrid effect of gas flow and light excitation in carbon/silicon Schottky solar cells. J. Mater. Chem. 2012, 22, 3330–3334. [Google Scholar] [CrossRef]
- Somani, P.R. Pressure sensitive multifunctional solar cells using carbon nanotubes. Appl. Phys. Lett. 2010, 96, 173504. [Google Scholar] [CrossRef]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.C. Gas sensor array based on metal-decorated carbon nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef]
- Wang, Z.L.; Chen, J.; Lin, L. Progress in triboelectric nanogenerators as a new energy technology and self-powered sensors. Energy Environ. Sci. 2015, 8, 2250–2282. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, W.Z. Nanotechnology-enabled energy harvesting for self-powered micro-/nanosystems. Angew. Chem. Int. Edit. 2012, 51, 11700–11721. [Google Scholar] [CrossRef]
- Chiesa, M.; Rigoni, F.; Paderno, M.; Borghetti, P.; Gagliotti, G.; Bertoni, M.; Ballarin Denti, A.; Schiavina, L.; Goldoni, A.; Sangaletti, L. Development of low-cost ammonia gas sensors and data analysis algorithms to implement a monitoring grid of urban environmental pollutants. J. Environ. Monit. 2012, 14, 1565–1575. [Google Scholar] [CrossRef]
- Backes, A.M.; Aulinger, A.; Bieser, J.; Matthias, V.; Quante, M. Ammonia emissions in Europe, part II: How ammonia emission abatement strategies affect secondary aerosols. Atmos. Environ. 2016, 126, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Burkhardt, J.; Sutton, M.A.; Milford, C.; Storeton West, L.R.; Fowler, D. Ammonia Concentrations at a site in Southern Scotland from 2-yr of continuous measurements. Atmos. Environ. 1998, 32, 325–331. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Di Menno Di Bucchianico, A.; Allegrini, I. Gaseous Ammonia in the urban area of Rome, Italy, and its relationship with traffic emissions. Atmos. Environ. 2002, 36, 5385–5394. [Google Scholar] [CrossRef]
- Marcazzan, G.M.; Vaccaro, S.; Valli, G.; Vecchi, R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy). Atmos. Environ. 2001, 35, 4639–4650. [Google Scholar] [CrossRef]
- Marcazzan, G.M.; Ceriani, M.; Valli, G.; Vecchi, R. Source apportionment of PM10 and PM2.5 in Milan (Italy) using receptor modelling. Sci. Total Environ. 2003, 317, 137–147. [Google Scholar] [CrossRef]
- Baek, B.H.; Aneya, V.P.; Tong, Q. Chemical coupling between ammonia, acid gases, and fine particles. Environ. Pollut. 2004, 129, 89–98. [Google Scholar] [CrossRef]
- Santini, G.; Mores, N.; Penas, A.; Capuano, R.; Mondino, C.; Trové, A.; Macagno, F.; Zini, G.; Cattani, P.; Martinelli, E.; et al. Electronic Nose and Exhaled Breath NMR-based Metabolomics Applications in Airways Disease. Curr. Top. Med. Chem. 2016, 16, 1610–1630. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Chuang, M.-Y.; Chen, C.C.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J. Organic Gas Sensor with an Improved Lifetime for Detecting Breath Ammonia in Hemodialysis Patients. ACS Sens. 2017, 2, 1788–1795. [Google Scholar] [CrossRef]
- Narasimhan, L.R.; Goodman, W.; Kumar, C.; Patel, N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. USA 2001, 98, 4617–4621. [Google Scholar] [CrossRef] [Green Version]
- Popa, C.; Dutu, D.C.; Cernat, R.; Matei, C.; Bratu, A.M.; Banita, S.; Dumitras, D.C. Ethylene and ammonia traces measurements from the patients’ breath with renal failure via LPAS method. Appl. Phys. B 2011, 105, 669–674. [Google Scholar] [CrossRef]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak-Lozowska, M.M.; Zietkowski, Z.; Przeslaw, K.; Tomasiak, M.; Skiepko, R.; Bodzenta-Lukaszyk, A. Inflammatory Markers and Acid-Base Equilibrium in Exhaled Breath Condensate of Stable and Unstable Asthma Patients. Int. Arch. Allergy Immunol. 2012, 159, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Zhang, Q.; Chow, C.L.; Tan, O.K.; Marzari, N. Sensing mechanisms for carbon nanotube based NH3 gas detection. Nano Lett. 2009, 9, 1626–1630. [Google Scholar] [CrossRef]
- Nguyen, L.Q.; Phan, P.Q.; Duong, H.N.; Nguyen, C.D.; Nguyen, L.H. Enhancement of NH3 Gas Sensitivity at Room Temperature by Carbon Nanotube-Based Sensor Coated with Co Nanoparticles. Sensors 2013, 13, 1754–1762. [Google Scholar] [CrossRef] [Green Version]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Rigoni, F.; Freddi, S.; Pagliara, S.; Drera, G.; Sangaletti, L.; Suisse, J.M.; Bouvet, M.; Malavichko, A.M.; Emelianov, A.V.; Bobrinetskiy, I.I. Humidity-enhanced sub-ppm sensitivity to ammonia of covalently functionalized single-wall carbon nanotube bundle layers. Nanotechnology 2017, 28, 255502. [Google Scholar] [CrossRef]
- Akbari, E.; Buntat, Z.; Ahmad, M.H.; Enzevaee, A.; Yousof, R.; Iqbal, S.M.Z.; Taghi Ahmadi, M.; Sidik, M.A.B.; Karimi, H. Analytical Calculation of Sensing Parameters on Carbon Nanotube Based Gas Sensors. Sensors 2014, 14, 5502–5515. [Google Scholar] [CrossRef] [Green Version]
- Goldoni, A.; Alijani, V.; Sangaletti, L.; D’Arsiè, L. Advanced promising routes of carbon/metal oxides hybrids in sensors: A review. Electrochim. Acta 2018, 266, 139–150. [Google Scholar] [CrossRef]
- Freddi, S.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Enhanced selectivity of target gas molecules through a minimal array of gas sensors based on nanoparticle-decorated SWCNTs. Analyst 2019, 144, 4100–4110. [Google Scholar] [CrossRef]
- Rigoni, F.; Tognolini, S.; Borghetti, P.; Drera, G.; Pagliara, S.; Goldoni, A.; Sangaletti, L. Environmental monitoring of low-ppb ammonia concentrations based on single-wall carbon nanotube chemiresistor gas sensors: Detection limits, response dynamics, and moisture effects. Procedia Eng. 2014, 87, 716–719. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-Performance Gas Sensor of Polyaniline/Carbon Nanotube Composites Promoted by Interface Engineering. Sensors 2020, 20, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nicola, F.; Salvato, M.; Cirillo, C.; Crivellari, M.; Boscardin, M.; Scarselli, M.; Nanni, F.; Cacciotti, I.; De Crescenzi, M.; Castrucci, P. Record efficiency of air stable multi-walled carbon nanotube/silicon solar cells. Carbon 2016, 101, 226–234. [Google Scholar] [CrossRef] [Green Version]
- De Nicola, F.; Salvato, M.; Cirillo, C.; Crivellari, M.; Boscardin, M.; Passacantando, M.; Nardone, M.; De Matteis, F.; Motta, N.; De Crescenzi, M.; et al. 100% internal quantum efficiency in polychiral single-walled carbon nanotube bulk heterojunction/silicon solar cells. Carbon 2017, 114, 402–410. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Adu, C.K.W.; Fang, S.; Eklund, P.C. Effects of Gas Adsorption and Collisions on Electrical Transport in Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2000, 85, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhi, S.H.; Louie, S.G.; Cohen, M.L. Electronic Properties of Oxidized Carbon Nanotubes. Phys. Rev. Lett. 2000, 85, 1710. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef]
- Kang, D.; Park, N.; Ko, J.; Bae, E.; Park, W. Oxygen-induced p-type doping of a long individual single-walled carbon nanotube. Nanotechnology 2005, 16, 1048–1052. [Google Scholar] [CrossRef]
- Freddi, S.; Achilli, S.; Soave, R.; Pagliara, S.; Drera, G.; De Poli, A.; De Nicola, F.; De Crescenzi, M.; Castrucci, P.; Sangaletti, L. Dramatic efficiency boost of single-walled carbon nanotube-silicon hybrid solar cells through exposure to ppm nitrogen dioxide in air: An ab-initio assessment of the measured device performances. J. Colloid Interf. Sci. 2020, 566, 60–68. [Google Scholar] [CrossRef]
- Sheindorf, C.; Rebhun, M.; Sheintuch, M. A Freundlich-type multicomponent isotherm. J. Colloid Interf. Sci. 1981, 79, 136–142. [Google Scholar] [CrossRef]
- Da Rocha, M.S.; Iha, K.; Faleiros, A.C.; Corat, E.J.; Suàrez-Iha, M.E.V. Freundlich’s isotherm extended by statistical mechanics. J. Colloid Interf. Sci. 1997, 185, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.S.; Cho, K.; Qi, P.; Dai, H. Ab initio study of CNT NO2 gas sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Ponzoni, S.; Achilli, S.; Pintossi, C.; Drera, G.; Sangaletti, L.; Castrucci, P.; De Crescenzi, M.; Pagliara, S. Hybridized C–O–Si Interface States at the Origin of Efficiency Improvement in CNT/Si Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 16627–16634. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A. Solar Cells Operating Principles; Prentice Hall: Englewood Cliffs, NJ, USA, 1982. [Google Scholar]






| [NH3] | CRD (ΔR/R0, %) | CRL (ΔR/R0, %) | PV (ΔPmax/P, %) |
|---|---|---|---|
| 1.0 ppm | 1.5 | 2.6 | 10 |
| 4.5 ppm | 2.6 | 5.6 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drera, G.; Freddi, S.; Freddi, T.; De Poli, A.; Pagliara, S.; De Crescenzi, M.; Castrucci, P.; Sangaletti, L. Gas Sensing with Solar Cells: The Case of NH3 Detection through Nanocarbon/Silicon Hybrid Heterojunctions. Nanomaterials 2020, 10, 2303. https://doi.org/10.3390/nano10112303
Drera G, Freddi S, Freddi T, De Poli A, Pagliara S, De Crescenzi M, Castrucci P, Sangaletti L. Gas Sensing with Solar Cells: The Case of NH3 Detection through Nanocarbon/Silicon Hybrid Heterojunctions. Nanomaterials. 2020; 10(11):2303. https://doi.org/10.3390/nano10112303
Chicago/Turabian StyleDrera, Giovanni, Sonia Freddi, Tiziano Freddi, Andrea De Poli, Stefania Pagliara, Maurizio De Crescenzi, Paola Castrucci, and Luigi Sangaletti. 2020. "Gas Sensing with Solar Cells: The Case of NH3 Detection through Nanocarbon/Silicon Hybrid Heterojunctions" Nanomaterials 10, no. 11: 2303. https://doi.org/10.3390/nano10112303