Preparation and Photocatalytic Performance for Degradation of Rhodamine B of AgPt/Bi4Ti3O12 Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bi4Ti3O12 Powders
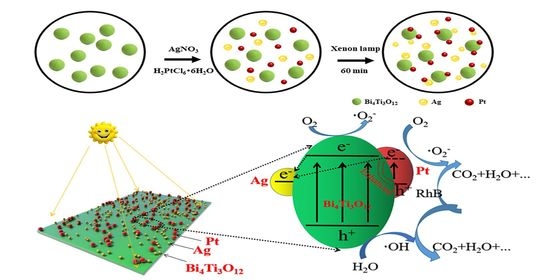
2.3. Assembly of Ag and Pt Nanoparticles (NPs) on Bi4Ti3O12 Nanosheets
2.4. Characterization
2.5. Photocatalytic Activity
2.6. Detection of Reactive Species
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alansi, A.M.; Al-Qunaibit, M.; Alade, I.O.; Qahtan, T.F.; Saleh, T.A. Visible-light responsive BiOBr nanoparticles loaded on reduced graphene oxide for photocatalytic degradation of dye. J. Mol. Liq. 2018, 253, 297–304. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Mohamed, A.R. A review on photocatalytic application of g-C3N4/semiconductor (CNS) nanocomposites towards the erasure of dyeing wastewater. Mater. Sci. Semicond. Process. 2016, 47, 62. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Chávez, A.; Gimeno, O.; Rey, A.; Pliego, G.; Oropesa, A.; Álvarez, P.; Beltrán, F. Treatment of highly polluted industrial wastewater by means of sequential aerobic biological oxidation-ozone based AOPs. Chem. Eng. J. 2019, 361, 89–98. [Google Scholar] [CrossRef]
- Do, M.H.; Phan, N.H.; Nguyen, T.K.P.; Pham, T.T.S.; Nguyen, V.K.; Vu, T.T.T. Activated carbon/Fe3O4 nanoparticle composite: Fabrication, methyl orange removal and regeneration by hydrogen peroxide. Chemosphere 2011, 85, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Murugesan, B.; Loganathan, A. A review on decolourisation of dyes by photodegradation using various bismuth catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 2300–2306. [Google Scholar] [CrossRef]
- Mahmood, T.; Wang, X.S.; Chen, C.C.; Ma, W.H. Photocatalytic degradation of persistent and toxic organic pollutants. Chin. J. Catal. 2007, 21, 1117. [Google Scholar] [CrossRef]
- Xie, J.; Guo, N.; Liu, A.; Cao, Y.; Hu, J.; Jia, D. Simple solid-state synthesis of BiOCl/Bi2O2CO3 heterojunction and its excellent photocatalytic degradation of RhB. J. Alloys Compd. 2019, 784, 377–385. [Google Scholar] [CrossRef]
- Ji, Y.C.; Yang, R.Q.; Wang, L.W.; Song, G.X.; Wang, A.Z.; Lv, Y.W.; Gao, M.M.; Zhang, J.; Yu, X. Visible light active and noble metal free Nb4N5/TiO2 nanobelt surface heterostructure for plasmonic enhanced solar water splitting. Chem. Eng. J. 2020, 402, 126226. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Di, L.; Ma, J.; Zhang, H.; Dai, J. Photocatalytic reduction synthesis of SrTiO3-graphene nanocomposites and their enhanced photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.; Liu, D.; Zheng, X.; Fu, X. N-Doped Carbon-Coated ZnS with Sulfur-Vacancy Defect for Enhanced Photocatalytic Activity in the Visible Light Region. Nanomaterials 2019, 9, 1657. [Google Scholar] [CrossRef] [Green Version]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.Y.; Li, L.; Zhang, X.T. In situ ion exchange synthesis of the Bi4Ti3O12/Bi2S3 heterostructure with enhanced photocatalytic activity. Catal. Commun. 2015, 60, 23. [Google Scholar] [CrossRef]
- Shen, G.D.; Pu, Y.P.; Sun, R.J.; Shi, Y.; Cui, Y.F.; Jing, P.P. Enhanced visible light photocatalytic performance of a novel heterostructured Bi4Ti3O12/BiOBr photocatalyst. New J. Chem. 2019, 43, 1. [Google Scholar] [CrossRef]
- Zheng, C.X.; Yang, H.; Cui, Z.; Zhang, H.M.; Wang, X.X. A novel Bi4Ti3O12/Ag3PO4 heterojunction photocatalyst with enhanced photocatalytic performance. Nanoscale Res. Lett. 2017, 12, 608. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Fan, W.; Geng, Z.; Feng, L. Enhancement of the photocatalytic performance of Ni-loaded TiO2 photocatalyst under sunlight. Ceram. Int. 2014, 40, 3887–3893. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Liu, Y.; Wang, J.; Ji, G. Fabrication and enhanced photocatalytic properties of Pt@SiO2@TiO2 composites by surface plasma resonance from Pt nanoparticles. J. Nanopart. Res. 2015, 17, 62. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lai, M.; Fang, J.J.; Lu, C.H. Au and Pt selectively deposited on {001}-faceted TiO2 toward SPR enhanced photocatalytic Cr(VI) reduction: The influence of excitation wavelength. Appl. Surf. Sci. 2018, 439, 430–438. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Q.; Ge, L.; Yin, L.; Fan, B.; Wang, H.; Lu, H.; Xu, H.; Zhang, R.; Shao, G. Synthesis and Ag-loading-density-dependent photocatalytic activity of Ag@TiO2 hybrid nanocrystals. Appl. Surf. Sci. 2013, 284, 921–929. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Wang, J.; Li, S.; Zhang, H.; Zhang, S. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Li, J.Y.; Tian, L.; Liang, F.; Wang, J.K.; Han, L.; Zhang, J.; Ge, S.T.; Dong, L.H.; Zhang, H.J.; Zhang, S.W. Molten salt synthesis of hierarchical porous N-doped carbon submicrospheres for multifunctional applications: High performance supercapacitor, dye removal and CO2 capture. Carbon 2019, 141, 739. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Liang, F.; Chang, S.; Zhang, H.; Zhang, M.; Zhang, S. Facile molten salt synthesis of atomically thin boron nitride nanosheets and their co-catalytic effect on the performance of carbon nitride photocatalyst. J. Colloid Interface Sci. 2019, 536, 664–672. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Huang, L.; Tian, L.; Wang, S.; Zhang, G.; Li, J.; Liang, F.; Zhang, H.; Jia, Q.; et al. One-step synthesis of dandelion-like lanthanum titanate nanostructures for enhanced photocatalytic performance. NPG Asia Mater. 2020, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Duan, H.; Liu, J.; Zhang, H. Preparation of lanthanum cerate powders via a simple molten salt route. Ceram. Int. 2016, 42, 10482–10486. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.-F.; Sun, H.; Jia, F.-C.; Kumar, P.; Liu, B. Synthesis and room-temperature H2S sensing of Pt nanoparticle-functionalized SnO2 mesoporous nanoflowers. J. Alloys Compd. 2020, 842, 155813. [Google Scholar] [CrossRef]
- Lin, C.; Ma, C.Q.; Yi, F.T.; Zhang, H.N.; Qian, Y.X.; Zhang, K.F. Ag NPs modified plasmonic Z-scheme photocatalyst Bi4Ti3O12/Ag/Ag3PO4 with improved performance for pollutants removal under visible light irradiation. Ceram. Int. 2020, 46, 14650–14661. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Li, S.; Cui, Z.; Zhang, C. Synthesis and theoretical study of large-sized Bi4Ti3O12 square nanosheets with high photocatalytic activity. Mater. Res. Bull. 2018, 107, 180–188. [Google Scholar] [CrossRef]
- Pooladi, M.; Shokrollahi, H.; Lavasani, S.; Yang, H. Investigation of the structural, magnetic and dielectric properties of Mn-doped Bi2Fe4O9 produced by reverse chemical co-precipitation. Mater. Chem. Phys. 2019, 229, 39–48. [Google Scholar] [CrossRef]
- Du, C.; Li, D.H.; He, Q.Y.; Liu, J.M.; Li, W.; He, G.N.; Wang, Y.Z. Design and simple synthesis of composite Bi12TiO20/Bi4Ti3O12 with a good photocatalytic quantum efficiency and high production of photo-generated hydroxyl radicals. Phys. Chem. Chem. Phys. Pccp. 2016, 18, 26530. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Hu, H.; Zhang, Q.; Tao, D. Mechanochemical synthesis of novel Pt modified ZnAl-LDH for effective ciprofloxacin photodegradation. J. Solid State Chem. 2020, 290, 121594. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Cui, Z.; Yi, Z.; Yu, H. Synergistically enhanced photocatalytic performance of Bi4Ti3O12 nanosheets by Au and Ag nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 13785–13796. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Sharma, S.K.; Abinnas, N.; Durgadevi, E.; Raji, P.; Muthamizhchelvan, C.; Ponnusamy, S.; Hayakawa, Y.; Kim, D.Y. Fabrication of the flexible nanogenerator from BTO nanopowders on graphene coated PMMA substrates by sol-gel method. Mater. Chem. Phys. 2017, 192, 274–281. [Google Scholar] [CrossRef]
- Lin, X.; Guan, Q.F.; Liu, T.T.; Zhang, Y.; Zou, C.J. Controllable synthesis and photocatalytic activity of Bi4Ti3O12 particles with different morphologies. Acta Phys. Chim. Sin. 2013, 29, 411–417. [Google Scholar]
- Liu, H.; Wu, R.; Tian, L.; Kong, Y.; Sun, Y. Synergetic photocatalytic effect between 1 T@2H-MoS2 and plasmon resonance induced by Ag quantum dots. Nanotechnology 2018, 29, 285402. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Hou, F.; Li, H.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Sun, K.; Shen, J.; Liu, Q.; Tang, H.; Zhang, M.; Zulfiqar, S.; Lei, C. Synergistic effect of Co(II)-hole and Pt-electron cocatalysts for enhanced photocatalytic hydrogen evolution performance of P-doped g-C3N4. Chin. J. Catal. 2020, 41, 72–81. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhou, X.; Jiang, H.; Yang, H.G.; Li, C. Positively charged Pt-based cocatalysts: An orientation for achieving efficient photocatalytic water splitting. J. Mater. Chem. A 2020, 8, 17–26. [Google Scholar] [CrossRef]
- Lin, D.; Gao, M.; You, L. Fabrication of novel Ag/AgVO3/WO3 homojunction/heterojunction nanomaterials with highly enhanced photocatalytic activity-Investigation on type plus Z-scheme mechanism. J. Alloys Compd. 2020, 846, 156274. [Google Scholar] [CrossRef]
- Shen, X.; Yang, J.; Zheng, T.; Wang, Q.; Zhuang, H.; Zheng, R.; Shan, S.; Li, S. Plasmonic p-n heterojunction of Ag/Ag2S/Ag2MoO4 with enhanced Vis-NIR photocatalytic activity for purifying wastewater. Sep. Purif. Technol. 2020, 251, 117347. [Google Scholar] [CrossRef]
- Chang, M.J.; Cui, W.N.; Liu, J.; Wang, K.; Luo, Z.M. Construction of novel TiO2/Bi4Ti3O12/MoS2 core/shell nanofibers for enhanced visible light photocatalysis. J. Mater. Sci. Technol. 2020, 36, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Gao, J.; Hojamberdiev, M.; Zhu, R.; Wei, X.; Guo, Q.; Liu, P. Enhanced photocatalytic activity of Bi4Ti3O12 nanosheets by Fe3+-doping and the addition of Au nanoparticles: Photodegradation of Phenol and bisphenol A. Appl. Catal. B Environ. 2017, 200, 72–82. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X.; Zhu, C.; Shi, C. Chromium-modified Bi4Ti3O12 photocatalyst: Application for hydrogen evolution and pollutant degradation. Appl. Catal. B Environ. 2016, 199, 241–251. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Cui, Z.; Li, R.; Feng, W. Enhanced photocatalytic performance of Ag–Bi4Ti3O12 nanocomposites prepared by a photocatalytic reduction method. Mater. Technol. 2017, 32, 870–880. [Google Scholar] [CrossRef]
- Chen, Z.W.; Jiang, H.; Jin, W.L.; Shi, C.K. Enhanced photocatalytic performance over Bi4Ti3O12 nanosheets with controllable size and exposed {001} facets for Rhodamine B degradation. Appl. Catal. B 2016, 180, 698. [Google Scholar] [CrossRef]
- Dutta, D.P.; Tyagi, A. Facile sonochemical synthesis of Ag modified Bi4Ti3O12 nanoparticles with enhanced photocatalytic activity under visible light. Mater. Res. Bull. 2016, 74, 397–407. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.H.; Gao, Z.Q.; Zhu, X.; Liu, Y.; Wei, Z.B.; Zhao, W.; Sun, C. A simple and effective method for fabricating novel p-n heterojunction photocatalyst g-C3N4/Bi4Ti3O12 and its photocatalytic performances. Appl. Catal. B 2016, 192, 57. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Wu, W.; Deng, Y.; Xiang, Y. A Novel Rose-Like CuS/Bi2WO6 Composite for Rhodamine B Degradation. ChemistrySelect 2019, 4, 11853–11861. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Tahiri, H.; Ait-Ichou, Y.; Lassaletta, G.; González-Elipe, A.R.; Fernández, A. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B Environ. 1997, 13, 219–228. [Google Scholar] [CrossRef]
- Low, J.; Qiu, S.; Xu, D.; Jiang, C.; Cheng, B. Direct evidence and enhancement of surface plasmon resonance effect on Ag-loaded TiO2 nanotube arrays for photocatalytic CO2 reduction. Appl. Surf. Sci. 2018, 434, 423–432. [Google Scholar] [CrossRef]
- Yan, T.; Tian, J.; Guan, W.; Qiao, Z.; Li, W.; You, J.; Huang, B. Ultra-low loading of Ag3PO4 on hierarchical In2S3 microspheres to improve the photocatalytic performance: The cocatalytic effect of Ag and Ag3PO4. Appl. Catal. B Environ. 2017, 202, 84–94. [Google Scholar] [CrossRef]
- Gao, J.; Ren, X.; Chen, D.; Tang, F.; Ren, J. Bimetallic Ag–Pt hollow nanoparticles: Synthesis and tunable surface plasmon resonance. Scr. Mater. 2007, 57, 687–690. [Google Scholar] [CrossRef]













| Firing Conditions | Salt Medium Composition (Molar Ratio) | Mass Ratio of Salt to Reactant |
|---|---|---|
| 700 °C/2 h | NaCl:KCl (1:1) | 1:1 |
| 800 °C/2 h | ||
| 900 °C/2 h | ||
| 700 °C/2 h | NaCl:KCl (1:1) | 2:1 |
| 800 °C/2 h | ||
| 900 °C/2 h | ||
| 700 °C/2 h | NaCl:KCl (1:1) | 3:1 |
| 800 °C/2 h | ||
| 900 °C/2 h |
| Metal Composition (Mass Ratio) | Loading Capacity | Illumination Condition |
|---|---|---|
| Ag | 0.1 wt% | 300 W xenon lamp (λ > 400 nm) 60 min |
| 0.2 wt% | ||
| 0.5 wt% | ||
| 1.0 wt% | ||
| 3.0 wt% | ||
| 5.0 wt% | ||
| Pt | 0.1 wt% | |
| Ag:Pt (1:1) | 0.1 wt% | |
| Ag:Pt (7:3) | ||
| Ag:Pt (3:7) | ||
| Ag:Pt (9:1) | ||
| Ag:Pt (1:9) |
| As-Prepared Photocatalyst | Element | Content (wt%) |
|---|---|---|
| Ag0.001/Bi4Ti3O12 | Ag | 0.0347 |
| Pt0.001/Bi4Ti3O12 | Pt | 0.0782 |
| (Ag0.7Pt0.3)0.001/Bi4Ti3O12 | Ag | 0.0122 |
| Pt | 0.0144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Zhang, G.; Li, K.; Li, F.; Cao, Y.; He, J.; Huang, Z.; Jia, Q.; Zhang, S.; Zhang, H. Preparation and Photocatalytic Performance for Degradation of Rhodamine B of AgPt/Bi4Ti3O12 Composites. Nanomaterials 2020, 10, 2206. https://doi.org/10.3390/nano10112206
Yuan G, Zhang G, Li K, Li F, Cao Y, He J, Huang Z, Jia Q, Zhang S, Zhang H. Preparation and Photocatalytic Performance for Degradation of Rhodamine B of AgPt/Bi4Ti3O12 Composites. Nanomaterials. 2020; 10(11):2206. https://doi.org/10.3390/nano10112206
Chicago/Turabian StyleYuan, Gaoqian, Gen Zhang, Kezhuo Li, Faliang Li, Yunbo Cao, Jiangfeng He, Zhong Huang, Quanli Jia, Shaowei Zhang, and Haijun Zhang. 2020. "Preparation and Photocatalytic Performance for Degradation of Rhodamine B of AgPt/Bi4Ti3O12 Composites" Nanomaterials 10, no. 11: 2206. https://doi.org/10.3390/nano10112206