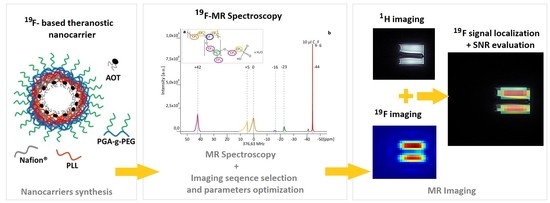
1. Introduction
Since the results of the first study demonstrating the feasibility of fluorine magnetic resonance imaging (
19F MRI) were published in 1977 [
1], rapid advances in both MRI hardware and software allowed for the development and investigation of various applications of
19F MRI. The main research directions included the imaging of fluorinated drugs, cell tracking, inflammation imaging and cancer diagnostics, the developing of oxygen-sensing and -responsive probes, and designing targeted fluorinated imaging agents. Those applications were extensively described and their results were summarized in many reviews [
2,
3,
4,
5,
6].
There are several preferable physical, chemical and biological properties making
19F MRI a potentially promising method for tracing theranostic agents—i.e., with combined therapeutic and diagnostic functionality. Gyromagnetic ratio of
19F nucleus is very close to the
1H value (40.08 vs. 42.58 MHz/T), which means that those spins process almost at the same resonance frequency (376.75 vs. 400.25 MHz at 9.4 T), allowing for the use of
1H MR hardware and software instrumentation with only a minimal adjustment for the
19F imaging [
5]. Moreover,
19F has a natural abundance of 100%, resulting in nuclear magnetic resonance (NMR) receptivity (an overall NMR sensitivity of a nucleus at natural abundance) of 0.83 relative to
1H. The close to zero natural concentration of
19F nuclei in the human body makes fluorine atoms a perfect MRI marker without any natural background signal. This creates the opportunity of localizing and identifying only exogenous fluorinated compounds with 100% specificity of the signal, assuring excellent contrast-to-noise ratio. To track the distribution of
19F labeled therapeutics in an anatomical context, the
19F MRI results are overlaid over the conventional
1H image acquired in the same imaging session
Nafion
® is a fluorinated polymer—high molecular weight, negatively charged molecule—which is relatively easy to incorporate it into nanocapsule shell via the layer by layer method [
7,
8]. From a synthesis point of view, this creates an excellent opportunity for designing a highly repetitive and efficient process for the production of theranostic nanocarriers for drug delivery. However, taking into consideration MR imaging, there are several issues making the visualization of the spatial distribution of Nafion
®-containing nanocarriers in the sample challenging. Those challenges are complex, multipeak
19F NMR spectrum composed of relatively broad lines in order of 1–2 ppm which correspond to short (in range of single milliseconds) transverse magnetization decay.
The occurrence of multiple resonances in the
19F MR spectrum leads to chemical shift artifacts in
19F images, i.e., multiple, spatially shifted images in frequency encoding direction. While the simplest approach to avoid it is choosing molecules with multiple identical fluorine atoms, such as perfluoro-15-crown-5-ether (PFCE), using them it is not always straightforward due to their chemical properties. Alternatively, methods for compensation of unwanted resonance signals can be applied and, in some cases, if one of the resonances is separated enough from others, the compensation may be entirely omitted [
9,
10,
11].
As many of the available
19F compounds exhibit short (in range of single milliseconds) transverse relaxation time, the choice of an adequate imaging sequence is of the utmost importance. There are only a few commercially available sequences allowing for the effective imaging of
19F fast-relaxing components. Those include the ultrashort echo time (UTE) or its 3D implementation (3D UTE) and the zero-echo time (ZTE) imaging sequences. Both sequences allow for the preservation of the signal from rapid-relaxing components, as they require very little time for preparation, maximizing the time for sampling [
12,
13]. Therefore, the optimization of imaging parameters of such sequences allows for the visualization of
19F compounds with less preferable characteristics.
The present work has aimed to assess the possibility to use 3D UTE pulse sequences (
Figure 1) at a high magnetic field for visualization of the distribution of
19F loaded theranostic nanocapsules with Nafion
® molecules incorporated into the shell. The presented work and [
8] present complementary parts of the research. While [
8] is focused on nanocapsule synthesis and characterization, this paper provides the extensive description of MR imaging and spectroscopy experiments for both Nafion
® solution and Nafion
®-loaded nanocapsules, which were accomplished in order to optimize the
19F MRI-based visualization of the nanocapsules distribution.
2. Materials and Methods
Nafion
® polymer (1,1,2,2-tetrafluoroethene;1,1,2,2-tetrafluoro-2-[1,1,1,2,3,3-hexafluoro-3- (1,2,2-trifluoroethenoxy)propan-2-yl] oxyethanesulfonic acid) is anionic polymer that is synthesized by the copolymerization of a perfluorinated vinyl ether comonomer with tetrafluoroethylene (TFE), resulting in the chemical structure given in
Figure 2a [
14,
15].
The Nafion® molecule has five fluorine nuclei groups that give rise to multiple signals in the MR spectrum. The structure of a Nafion® unit illustrates the variability of the material—i.e., unreported by a vendor co-monomer distribution of molecular groups x and y that results in differences in 19F signal strength arising from a specified fluorine group from one synthesis to another.
The material used in experiments was Nafion® 20 wt. % solution in lower aliphatic alcohols and water (663492 Sigma-Aldrich; Poznań, Poland)). The stock solution was first used to acquire a full 19F MR spectrum and to measure T1 and T2 relaxation times. Next, a series of Nafion® dilutions in water with varied concentration were prepared to estimate the minimal number of 19F nuclei in a sample that can be visualized in reasonable scan time. Hexafluorobenzene purchased from Sigma Aldrich was used as a reference substance.
For theranostic nanocapsule synthesis, the polycation poly-L-lysine hydrobromide, PLL (MW 15 to 30 kDa) and polyanion: NAFION® (663492), as well as chloroform, sodium chloride and docusate sodium salt (AOT), were purchased from Sigma-Aldrich. All materials were used as received without further purification. The ultrapure water was produced using the Millipore Direct-Q5 UV Merck purification system.
All samples for MRI measurements were prepared in vials with micro-inserts containing a reference substance.
2.1. Polyelectrolyte Shell Liquid Core Nanocapsules Preparation and Characterization
The polyelectrolyte shell liquid core nanocapsules were prepared adopting the procedure proposed by us before [
7]—i.e., by the encapsulation of nanoemulsion droplets in the polyelectrolyte multilayer shell. The details of the preparation procedure are given in [
8]. Briefly, the oil phase for the nano emulsification was prepared by dissolving anionic surfactant AOT in chloroform at the concentration 340 g/L, while the water phase was prepared by dissolving poly-L-Lysine in 0.015 M NaCl solution (concentration varied from 10 to 300 ppm). The liquid core of capsules (nanoemulsion droplets) was formed by dispersing oil phase (0.1 mL) into the water phase (200 mL of polycation solution) during mixing with a magnetic stirrer at the rate 300 rpm. On such prepared liquid cores, the multilayer shell was formed by the subsequent adsorption of polyelectrolytes. After preparation, chloroform was evaporated with the final CHCl
3 concentration not exceeding 0.04 mg/L [
16].
2.2. MRI Equipment
Both 19F MR spectroscopy/relaxation measurements, as well as 1H and 19F 3DUTE imaging, were performed at the 9.4 T Bruker Biospec 94/20 research MRI scanner (Bruker Biospin, Ettlingen, Germany) with 210 mm bore diameter and high performance actively shielded BGA 12S HP gradient system (675 mT/m) with integrated shims. A small transmit-receive ribbon solenoid radiofrequency (RF) coil (ID of 14 mm), which can be tuned either to 1H or 19F resonant frequency (i.e., 400.130 vs. 376.498 MHz) was built and used for all experiments. Coil geometry was adjusted individually to analyzed sample size and shape to maximize the filling factor and thus Signal-To-Noise Ratio (SNR) values. Paravision 5.1 and Topspin 2.0 software was used to accomplish MR imaging and spectroscopy.
2.3. 19F Spectroscopy and Relaxometry
Prior to the imaging, an MR spectroscopy (with a 17 μs 90° pulse and SW: 59.52 kHz) and T1 and T2 measurements were performed in order to choose the peak with the largest area and consequently the highest NMR signal and to set imaging sequence parameters to values enabling an optimal visualization of Nafion® molecule distribution.
The T1 measurement was performed in TopSpin 2.0 (Bruker Biospin, Ettlingen, Germany) using the pseudo-2D version of a standard Inversion Recovery experiment exploiting an array of 10 different inversion recovery (IR) experiments with different inversion time values (0.15, 0.30, 0.75, 1.5, 3.0, 6.0 s). T2 values for individual resonances were estimated by calculating the reciprocal of their full width at half maximum (FWHM) values.
2.4. 3D UTE Pulse Sequence
Conventional MRI pulse sequences generate relatively long echo times (>> 1ms). Therefore, the majority of the signal from short transverse relaxation time components decays to near zero before echo formation or even during RF pulse excitation. The 3D Ultrashort TE sequence allows for measuring very short T
2 compounds, because of non-selective RF excitation pulse [
17,
18], and the minimum echo time (TE) is limited only by RF pulse duration and the time necessary to change between RF excitation and acquisition mode. A timing diagram for a 3D UTE sequence is presented in
Figure 1. Sampling is performed from the start of the rising gradient ramp; thus, it begins always in the center of a k-space and continues to the surface of a sphere. The image reconstruction is performed by regridding from radial k-space on a Cartesian grid followed by a conventional 3D fast Fourier transformation.
2.5. 3D UTE Imaging
The application of 3D UTE to actual experiments requires performing a preparation specific for this method, including gradient delay optimization and k-space trajectory measurement. The optimal gradient delay was determined using a glass sphere phantom filled with an aqueous solution of CuSO4 positioned in the isocenter of the scanner. Imaging with a gradient delay value of 1 µs resulted in the most homogenous image with the least apparent artifacts. The k-space trajectory was measured using the same phantom and stored to be used during actual imaging experiments. As the measured trajectory is valid only for specific scanning parameters (given matrix size, field of view, slice orientation, and acquisition bandwidth), thus this adjustment was repeated for each FOV and matrix size planned to be used in an imaging experiment.
The 3D UTE sequence was first applied to Nafion
® solutions of a different concentration. Imaging parameters were as follows: repetition time (TR): 8 ms, TE: 0.16 ms, RF pulse bandwidth (BW): 4.27 kHz, FOV: 4.0 × 4.0 × 4.0 cm
3. For
1H images: matrix size ( MTX): 128 × 128 × 128 and number of averages (NA):: 1, resulting in total acquisition time ∼6m 51s, while for
19F images: MTX: 32 × 32 × 32, NA: 64 and acquisition time: ∼27 m6 s. The flip angle (FA) was set to 6.4° according to the modified Ernst angle formula to account for the effects of a transverse relaxation during the RF excitation [
3].
Subsequently, the imaging of a phantom containing theranostic nano-carriers was performed with imaging parameters, as follows: TR: 8 ms, TE: 0.16 ms, FA: 6.4°, RF pulse BW: 4.27 kHz, FOV: 4.0 × 4.0 × 4.0 cm. For 1H images: MTX: 128 ×128 × 128 and NA: 1, acquisition time ∼ 6 m 51 s, while for 19F images: MTX: 32 × 32 × 32, NA: 256 or 500 and acquisition time: ∼ 1 h 48 min 24 s and ∼ 3 h 31 m 44 s, respectively.
4. Discussion
The most frequently reported substances in
19F MRI studies are perfluorocarbons (especially nanoemulsions of PFOB and PFCE). Depending on the chosen PFC and an MRI system that is used, the estimated number of
19F nuclei that can be visualized in a scan time below 1 hour is in the range of ∼10
16 of
19F nuclei/voxel [
19,
20]. Nonetheless, different chemical forms, such as liposomes, micelles or
19F modified implants, have also been investigated. The application of liposomal structures for drug delivery was shown by Bo et al. [
21]. The authors synthesized Doxorubicin (DOX)-loaded liposomes with high
19F content for use as
19F detectable drug-delivery at the therapeutic dose. They obtained a structure that resulted in a single
19F resonance line and a detection limit as low as 5 mmol of
19F for a scan time = 6 m 24 s. Other studies using various
19F copolymers for application as
19FMRI detectable tracers (spin-echo imaging with TE = 4.8 ms, TR = 3000 ms, and total imaging time restricted to 80 min) allowed the authors to obtain images with SNR values in the range from 3.9 to 6.9 (for samples with 2.3–9.3 mmol
19F/g concentration) [
22]. Yet, the total scan time of that range is one of the limiting factors to introduce those substances to clinical applications.
As the NMR signal is proportional to field strength, high field systems provide higher SNR than typical clinical scanners enabling the imaging of
19F nuclei at very low concentrations. It is desirable that tracers should be characterized by the high fluorine content, simple
19F NMR spectrum, with a single, sharp, and intense peak, short T
1 and long T
2 [
23]
. Nevertheless, due to advances in MRI instrumentation, and ultrashort echo pulse sequence development, the range of fluorine compounds available as a new MRI marker can be expanded, including one with less optimal characteristics, but easier to incorporate into theranostic nanocapsules. The use of high field equipment in preclinical imaging, as well as a tendency towards increasing the magnetic field of clinical MRI scanners, pave the way for use of a broader range of
19F compounds as a tracer.
As the signal from 19F compounds is usually only a little above the detection limits, the low SNR is the biggest challenge in 19F imaging. To achieve a sufficient SNR value, a compromise between spatial resolution and total scan time must be made. Collecting the signal with multiple averages causes an increase in signal intensity by the factor of , but at the same time, total scan time scales directly with NA. Another option to increase SNR is sampling a larger volume, which results in loss of spatial resolution. However, the use of a high-resolution 1H MR image as an anatomical framework for the presentation of the 19F-compound distribution alleviates this inconvenience.
In the presented work we managed to obtain 19F MR images of polyelectrolyte theranostic nanocapsules containing Nafion® polymer as a tracer, with SNR ≥ 5 and an acquisition time below 30 min, for 19F concentration as low as 1.53 × 10−2 mmol 19F/g of sample. This is comparable with the results obtained for molecules that are theoretically more suitable for application as 19F MRI tracers.
In our previous work, the effective contrasting properties of iron oxides and gadolinium-based compounds were effectively tested for the visualization of the distribution of the polyelectrolyte theranostic nanocapsules, using
1H MR imaging [
24,
25]. The possibility to use the
19F-containing Nafion
® compound, which is relatively easy to incorporate into a nanocapsule’s layers, broadens the potential applicability of these theranostic nanocarriers. The use of a 3D UTE pulse sequence at a high magnetic field of 9.4T allows for effective imaging of the presence of nanocapsules with Nafion
® incorporated into their shell. This indicates the possibility to use this approach in preclinical research, for the potential imaging of the in vivo distribution of nanocarriers, without a background signal, as is in the case with
1H MRI.