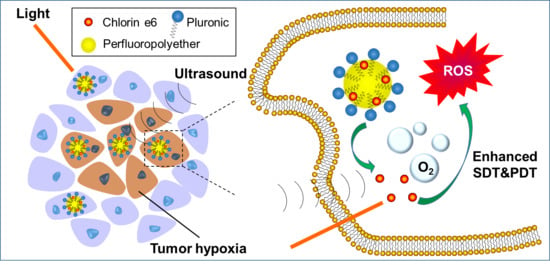
Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ce6-P/W NE
2.3. Characterization
2.4. Singlet Oxygen (1O2) Production in Response to Ultrasound or Light
2.5. Cell Culture
2.6. SDT Efficacy
2.7. PDT Efficacy
2.8. Cytocompatibility
2.9. Comparison between SDT and PDT on Tissue Penetration
3. Results and Discussion
3.1. Preparation and Characterization of an Oxygen-Carrying Photo-Sonotherapeutic (Ce6-P/W NE)
3.2. 1O2 Generation in Response to Ultrasound and Light Excitation
3.3. Enhanced SDT Efficacy
3.4. Enhanced PDT Efficacy
3.5. Cytocompatibility
3.6. Tissue-Penetrating Ability of SDT Compared with PDT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic therapy by in situ nonlinear photon conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]
- Zhu, J.; Chu, C.; Li, D.; Pang, X.; Zheng, H.; Wang, J.; Shi, Y.; Zhang, Y.; Cheng, Y.; Ren, E.; et al. Fe(III)-porphyrin sonotheranostics: A green triple-regulated ROS generation nanoplatform for enhanced cancer imaging and therapy. Adv. Funct. Mater. 2019, 29, 1904056. [Google Scholar] [CrossRef]
- Zhou, T.-J.; Xing, L.; Fan, Y.-T.; Cui, P.-F.; Jiang, H.-L. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J. Control. Release 2019, 307, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Mao, C. Enhancement of photodynamic cancer therapy by physical and chemical factors. Angew. Chem. Int. Ed. 2019, 58, 14066–14080. [Google Scholar] [CrossRef]
- He, Y.; Cong, C.; He, Y.; Hao, Z.; Li, C.; Wang, S.; Zhao, Q.; He, H.; Zhu, R.; Li, X.; et al. Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy. Chem. Eng. J. 2019, 375, 122079. [Google Scholar] [CrossRef]
- Wang, L.; Niu, M.; Zheng, C.; Zhao, H.; Niu, X.; Li, L.; Hu, Y.; Zhang, Y.; Shi, J.; Zhang, Z. A core–shell nanoplatform for synergistic enhanced sonodynamic therapy of hypoxic tumor via cascaded strategy. Adv. Healthc. Mater. 2018, 7, 1800819. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Wang, Q.; Hang, L.; Wang, Y.; Wang, Y. ROS-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy. Biomater. Sci. 2019, 7, 3706–3716. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Yu, Q.; Huang, T.C.; Liu, C.; Zhao, M.L.; Xie, M.J.; Li, G.; Liu, S.J.; Huang, W.; Zhao, Q. Oxygen self-sufficient NIR-activatable liposomes for tumor hypoxia regulation and photodynamic therapy. Chem. Sci. 2019, 10, 9091–9098. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef]
- Jia, T.; Xu, J.T.; Dong, S.M.; He, F.; Zhong, C.N.; Yang, G.X.; Bi, H.T.; Xu, M.S.; Hu, Y.K.; Yang, D.; et al. Mesoporous cerium oxide-coated upconversion nanoparticles for tumor-responsive chemo-photodynamic therapy and bioimaging. Chem. Sci. 2019, 10, 8618–8633. [Google Scholar] [CrossRef] [Green Version]
- Saggar, J.K.; Yu, M.; Tan, Q.; Tannock, I.F. The tumor microenvironment and strategies to improve drug distribution. Front. Oncol. 2013, 3, 154. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Zheng, D.; Li, B.; Xu, L.; Zhang, Q.-L.; Fan, J.-X.; Li, C.-X.; Zhang, X.-Z. Normalizing tumor microenvironment based on photosynthetic abiotic/biotic nanoparticles. ACS Nano 2018, 12, 6218–6227. [Google Scholar] [CrossRef]
- McEwan, C.; Owen, J.; Stride, E.; Fowley, C.; Nesbitt, H.; Cochrane, D.; Coussios, C.C.; Borden, M.; Nomikou, N.; McHale, A.P.; et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J. Control Release 2015, 203, 51–56. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.-S.; Zhang, W.; Wang, P. Photosensitizers for photodynamic therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Miyoshi, N.; Kundu, S.K.; Tuziuti, T.; Yasui, K.; Shimada, I.; Ito, Y. Combination of sonodynamic and photodynamic therapy against cancer would be effective through using a regulated size of nanoparticles. Nanosci. Nanoeng. 2016, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bhawalkar, J.D.; He, G.S.; Prasad, P.N. Nonlinear multiphoton processes in organic and polymeric materials. Rep. Prog. Phys. 1996, 59, 1041–1070. [Google Scholar] [CrossRef]
- Bhawalkar, J.D.; Kumar, N.D.; Zhao, C.F.; Prasad, P.N. Two-photon photodynamic therapy. J. Clin. Laser Med. Sur. 1997, 15, 201–204. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Ali, L.M.A.; Daurat, M.; Mattana, E.; Gary-Bobo, M. Biological assessment of laser-synthesized silicon nanoparticles effect in two-photon photodynamic therapy on breast cancer MCF-7 cells. Nanomaterials 2020, 10, 1462. [Google Scholar] [CrossRef]
- Gu, B.; Wu, W.; Xu, G.; Feng, G.; Yin, F.; Chong, P.H.J.; Qu, J.; Yong, K.-T.; Liu, B. Precise two-photon photodynamic therapy using an efficient photosensitizer with aggregation-induced emission characteristics. Adv. Mater. 2017, 29, 1701076. [Google Scholar] [CrossRef]
- Thorat, N.D.; Townely, H.; Brennan, G.; Parchur, A.K.; Silien, C.; Bauer, J.; Tofail, S.A.M. Progress in remotely triggered hybrid nanostructures for next-generation brain cancer theranostics. ACS Biomater. Sci. Eng. 2019, 5, 2669–2687. [Google Scholar] [CrossRef]
- Collins, H.A.; Khurana, M.; Moriyama, E.H.; Mariampillai, A.; Dahlstedt, E.; Balaz, M.; Kuimova, M.K.; Drobizhev, M.; Yang, V.X.D.; Phillips, D.; et al. Blood-vessel closure using photosensitizers engineered for two-photon excitation. Nat. Photonics 2008, 2, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Lafond, M.; Yoshizawa, S.; Umemura, S.-I. Sonodynamic therapy: Advances and challenges in clinical translation. J. Ultrasound. Med. 2019, 38, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/nanoparticle-augmented sonodynamic therapy (SDT): Breaking the depth shallow of photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Ziskin, M.C. Fundamental physics of ultrasound and its propagation in tissue. Radiographics 1993, 13, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Kim, H.-C.; Jung, Y.; Chung, Y.A.; Song, I.-U.; Lee, J.-H.; Yoo, S.-S. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep. 2016, 6, 34026. [Google Scholar] [CrossRef]
- Bystritsky, A.; Korb, A.S.; Douglas, P.K.; Cohen, M.S.; Melega, W.P.; Mulgaonkar, A.P.; DeSalles, A.; Min, B.K.; Yoof, S.S. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011, 4, 125–136. [Google Scholar] [CrossRef]
- Jolesz, F.A.; Hynynen, K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002, 8, S100–S112. [Google Scholar]
- Nomikou, N.; Curtis, K.; McEwan, C.; O’Hagan, B.M.G.; Callan, B.; Callan, J.F.; McHale, A.P. A versatile, stimulus-responsive nanoparticle-based platform for use in both sonodynamic and photodynamic cancer therapy. Acta Biomater. 2017, 49, 414–421. [Google Scholar] [CrossRef]
- Bilmin, K.; Kujawska, T.; Grieb, P. Sonodynamic therapy for gliomas. Perspectives and prospects of selective sonosensitization of glioma cells. Cells 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xu, T.; Huang, Q.; Jin, W.; Chen, J. Immunotherapy for malignant glioma: Current status and future directions. Trends Pharmacol. Sci. 2020, 41, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Chen, W.; Liu, L.; Yu, F. Light and sound to trigger the pandora’s box against breast cancer: A combination strategy of sonodynamic, photodynamic and photothermal therapies. Biomaterials 2020, 232, 119685. [Google Scholar] [CrossRef]
- Wang, L.H.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Daoudi, K.; van den Berg, P.J.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P. Steenbergen, Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Moseley, H.; Bown, S. Rationale of combined PDT and SDT modalities for treating cancer patients in terminal stage: The proper use of photosensitizer. Integr. Cancer Ther. 2010, 9, 317–319. [Google Scholar] [CrossRef] [Green Version]
- Sadanala, K.C.; Chaturvedi, P.K.; Seo, Y.M.; Kim, J.M.; Jo, Y.S.; Lee, Y.K.; Ahn, W.S. Sono-photodynamic combination therapy: A review on sensitizers. Anticancer Res. 2014, 34, 4657–4664. [Google Scholar]
- Wang, X.H.; Zhang, W.M.; Xu, Z.Y.; Luo, Y.F.; Mitchell, D.; Moss, R.W. Sonodynamic and photodynamic therapy in advanced breast carcinoma: A report of 3 cases. Integr. Cancer Ther. 2009, 8, 283–287. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Jiang, Y.; Wang, D. Multifunctional nanocapsules on a seesaw balancing sonodynamic and photodynamic therapies against superficial malignant tumors by effective immune-enhancement. Biomaterials 2019, 218, 119251. [Google Scholar] [CrossRef]
- Chen, J.; Luo, H.; Liu, Y.; Zhang, W.; Li, H.; Luo, T.; Zhang, K.; Zhao, Y.; Liu, J. Oxygen-self-produced nanoplatform for relieving hypoxia and breaking resistance to sonodynamic treatment of pancreatic cancer. ACS Nano 2017, 11, 12849–12862. [Google Scholar] [CrossRef] [PubMed]
- Chudal, L.; Pandey, N.K.; Phan, J.; Johnson, O.; Li, X.; Chen, W. Investigation of PPIX-Lipo-MnO2 to enhance photodynamic therapy by improving tumor hypoxia. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109979. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ni, N.; Ma, Y.; Wang, Y.; Leong, D.T. Retooling cancer nanotherapeutics’ entry into tumors to alleviate tumoral hypoxia. Small 2020. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Chen, H.C.; Tian, J.W.; He, W.J.; Guo, Z.J. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Deng, L.; Sheng, D.; Liu, M.; Yang, L.; Ran, H.; Li, P.; Cai, X.; Sun, Y.; Wang, Z. A near-infrared laser and H2O2 activated bio-nanoreactor for enhanced photodynamic therapy of hypoxic tumors. Biomater. Sci. 2020, 8, 858–870. [Google Scholar] [CrossRef]
- Luo, Z.; Zheng, M.; Zhao, P.; Chen, Z.; Siu, F.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Ma, Y.; et al. Self-monitoring artificial red cells with sufficient oxygen supply for enhanced photodynamic therapy. Sci. Rep. 2016, 6, 23393. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.Y.; Bai, H.T.; Liu, L.B.; Lv, F.T.; Ren, X.Q.; Wang, S. Luminescent, oxygen-supplying, hemoglobin-linked conjugated polymer nanoparticles for photodynamic therapy. Angew. Chem. Int. Ed. 2019, 58, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, B.; Wang, H.; Yuan, A.; Hu, Y.; Wu, J. Two-stage oxygen delivery for enhanced radiotherapy by perfluorocarbon nanoparticles. Theranostics 2018, 8, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef] [PubMed]
- Waxman, K. Perfluorocarbons as blood substitutes. Ann. Emerg. Med. 1986, 15, 1423–1424. [Google Scholar] [CrossRef]
- Choi, K.H.; Nam, K.C.; Cho, G.; Jung, J.S.; Park, B.J. Enhanced photodynamic anticancer activities of multifunctional magnetic nanoparticles (Fe3O4) conjugated with chlorin e6 and folic acid in prostate and breast cancer cells. Nanomaterials 2018, 8, 722. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Feng, L.; Liang, C.; Yang, K.; Liu, Z. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett. 2016, 16, 6145–6153. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Hu, D.R.; Chen, L.J.; Qu, Y.; Peng, J.R.; Chu, B.Y.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.Y.; Qian, Z.Y. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef]
- Woo, J.O.; Misran, M.; Lee, P.F.; Tan, L.P. Development of a controlled release of salicylic acid loaded stearic acid-oleic acid nanoparticles in cream for topical delivery. Sci. World J. 2014, 2014, 205703. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhou, C.L.; Chen, F.P.; Han, D.; Wang, C.Y.; Li, J.X.; Chi, Z.; Liu, C.G. Development of a carboxymethyl chitosan functionalized nanoemulsion formulation for increasing aqueous solubility, stability and skin permeability of astaxanthin using low-energy method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237. [Google Scholar] [CrossRef]
- Wik, J.; Bansal, K.K.; Assmuth, T.; Rosling, A.; Rosenholm, J.M. Facile methodology of nanoemulsion preparation using oily polymer for the delivery of poorly soluble drugs. Drug. Deliv. Transl. Res. 2020, 10, 1228–1240. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, M.; Wang, B.; Wang, P.; Liu, Y.; Zhao, Y.; Li, K.; Song, G.; Zhang, X.-B.; Tan, W. NIR-II driven plasmon-enhanced catalysis for a timely supply of oxygen to overcome hypoxia-induced radiotherapy tolerance. Angew. Chem. Int. Ed. 2019, 58, 15069–15075. [Google Scholar] [CrossRef]
- Deng, L.; Feng, Z.; Deng, H.; Jiang, Y.; Song, K.; Shi, Y.; Liu, S.; Zhang, J.; Bai, S.; Qin, Z.; et al. Rational design of nanoparticles to overcome poor tumor penetration and hypoxia-induced chemotherapy resistance: Combination of optimizing size and self-inducing high level of reactive oxygen species. ACS Appl. Mater. Interfaces 2019, 11, 31743–31754. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, F.; Gao, W.; Yang, X.; Ju, Y.; Zhao, L.; Guo, W.; Xie, J.; Liang, X.-J.; Tao, X.; et al. Magnetic reactive oxygen species nanoreactor for switchable magnetic resonance imaging guided cancer therapy based on pH-sensitive Fe5C2@Fe3O4 nanoparticles. ACS Nano 2019, 13, 10002–10014. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Caruthers, S.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann. Biomed. Eng. 2009, 37, 1922–1933. [Google Scholar] [CrossRef]
- Xu, F.; Hu, M.; Liu, C.; Choi, S.K. Yolk-structured multifunctional up-conversion nanoparticles for synergistic photodynamic–sonodynamic antibacterial resistance therapy. Biomater. Sci. 2017, 5, 678–685. [Google Scholar] [CrossRef]
- Jin, Z.H.; Miyoshi, N.; Ishiguro, K.; Umemura, S.; Kawabata, K.; Yumita, N.; Sakata, I.; Takaoka, K.; Udagawa, T.; Nakajima, S.; et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J. Dermatol. 2000, 27, 294–306. [Google Scholar] [CrossRef]
- Sun, Q.Q.; He, F.; Bi, H.T.; Wang, Z.; Sun, C.Q.; Li, C.X.; Xu, J.T.; Yang, D.; Wang, X.X.; Gai, S.L.; et al. An intelligent nanoplatform for simultaneously controlled chemo-, photothermal, and photodynamic therapies mediated by a single NIR light. Chem. Eng. J. 2019, 362, 679–691. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Hu, J.-J.; Qin, S.-Y.; Zhang, A.-Q.; Zhang, X.-Z. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 2020, 232, 119738. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.-G.; Cheng, K.; Li, C.; Hou, X.-L.; Wang, G.-L.; Zhang, X.-S.; Liu, B.; Zhao, Y.-D.; Zhang, M.-Z. ROS-augmented and tumor-microenvironment responsive biodegradable nanoplatform for enhancing chemo-sonodynamic therapy. Biomaterials 2020, 234, 119761. [Google Scholar] [CrossRef]
- Fu, J.; Li, T.; Zhu, Y.; Hao, Y. Ultrasound-activated oxygen and ROS generation nanosystem systematically modulates tumor microenvironment and sensitizes sonodynamic therapy for hypoxic solid tumors. Adv. Funct. Mater. 2019, 29, 1906195. [Google Scholar] [CrossRef]
- Jing, X.N.; Xu, Y.Z.; Liu, D.M.; Wu, Y.S.; Zhou, N.; Wang, D.Q.; Yan, K.; Meng, L.J. Intelligent nanoflowers: A full tumor microenvironment-responsive multimodal cancer theranostic nanoplatform. Nanoscale 2019, 11, 15508–15518. [Google Scholar] [CrossRef]
- Liu, P.; Xie, X.; Shi, X.; Peng, Y.; Ding, J.; Zhou, W. Oxygen-self-supplying and HIF-1α-inhibiting core–shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 11, 48261–48270. [Google Scholar] [CrossRef]
- Qiu, M.; Singh, A.; Wang, D.; Qu, J.L.; Swihart, M.; Zhang, H.; Prasad, P.N. Biocompatible and biodegradable inorganic nanostructures for nanomedicine: Silicon and black phosphorus. Nano Today 2019, 25, 135–155. [Google Scholar] [CrossRef]
- McEwan, C.; Nesbitt, H.; Nicholas, D.; Kavanagh, O.N.; McKenna, K.; Loan, P.; Jack, I.G.; McHale, A.P.; Callan, J.F. Comparing the efficacy of photodynamic and sonodynamic therapy in non-melanoma and melanoma skin cancer. Bioorg. Med. Chem. 2016, 24, 3023–3028. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.J.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C.; Ho, H.K.; Leong, D.T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Pacurari, M.; Qian, Y.; Fu, W.; Schwegler-Berry, D.; Ding, M.; Castranova, V.; Guo, N.L. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. J. Toxicol. Environ. Health Part A 2012, 75, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Tong, S.; Zhang, L.; Sakurai, Y.; Myers, D.R.; Hong, L.; Lam, W.A.; Bao, G. Magnetic forces enable controlled drug delivery by disrupting endothelial cell-cell junctions. Nat. Commun. 2017, 8, 15594. [Google Scholar] [CrossRef] [Green Version]
- Canavese, G.; Ancona, A.; Racca, L.; Canta, M.; Dumontel, B.; Barbaresco, F.; Limongi, T.; Cauda, V. Nanoparticle-assisted ultrasound: A special focus on sonodynamic therapy against cancer. Chem. Eng. J. 2018, 340, 155–172. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, L.; Pliss, A.M.; Zhan, Y.; Zheng, W.; Xia, J.; Liu, L.; Qu, J.; Prasad, P.N. Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor. Nanomaterials 2020, 10, 2058. https://doi.org/10.3390/nano10102058
Hong L, Pliss AM, Zhan Y, Zheng W, Xia J, Liu L, Qu J, Prasad PN. Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor. Nanomaterials. 2020; 10(10):2058. https://doi.org/10.3390/nano10102058
Chicago/Turabian StyleHong, Liang, Artem M. Pliss, Ye Zhan, Wenhan Zheng, Jun Xia, Liwei Liu, Junle Qu, and Paras N. Prasad. 2020. "Perfluoropolyether Nanoemulsion Encapsulating Chlorin e6 for Sonodynamic and Photodynamic Therapy of Hypoxic Tumor" Nanomaterials 10, no. 10: 2058. https://doi.org/10.3390/nano10102058