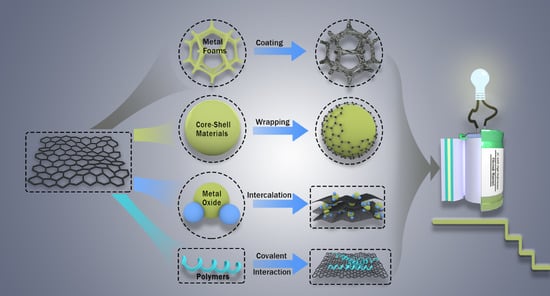
5.1. Metal-Based Electrodes
Many studies have reported various metal oxides mixed with graphene or rGO composites for the construction of electrochemical electrodes. The Mn
3O
4/graphene nanocomposites synthesized by Wang et al. exhibited a specific capacitance of 175 F/g in 1 M Na
2SO
4 and 256 F/g in 6 M KOH [
85]. He et al. synthesized CoFe
2O
4/rGO nanocomposite SC electrodes and obtained a specific capacitance of 123.3 F/g [
86]. Nagaraju et al. reported V
2O
5/rGO nanosheet electrodes possessing an impressive specific capacitance of 635 F/g at a current density of 1 A/g [
87]. Flower-like NiO/rGO synthesized by Li et al. exhibited a specific capacitance of 428 F/g at a current density of 0.38 A/g [
83]. Zhang et al. reported the synthesis of CdS/rGO nanocomposites and documented a specific capacitance of 300 F/g [
82,
83]. Metallic cellulose paper-based electrodes have been reported by Ko et al. for SC applications, which exhibited a maximum power of 15.1 mW cm
−2 [
88].
Most of the measurements of the aforementioned systems were carried out in the conventional three-electrode configuration. However, a review by Stoller and Ruoff suggested that the three-electrode configuration is valuable for the determination of electrochemical-specific material characteristics, while a two-electrode configuration portrays the physical configuration, charge transfer, and internal voltage of packaged SCs, thus providing better information on the electrode materials [
89]. Jiang et al. reported the in situ incorporation of GO flakes and PEDOT: PSS into the bacterial nanocellulose (BNC) matrix and their electrode materials exhibited a specific capacitance of 373 F/g at a current density of 1 A/g [
90]. Layered double hydroxides (LDHs) have recently been intensely studied as a candidate for SC applications. Wang et al. studied the performance of cobalt nickel iron–LDH/carbon nanofibers and activated carbon in asymmetric SCs, and reported their excellent performance [
91]. Peng et al. synthesized CoAl-LDH/fluorinated graphene composites and reported an even higher specific capacitance of 1222 F/g at 1 A/g, with a very good rate capability [
90,
91].
To date, various studies have been carried out by different research groups, which undoubtedly confirmed the huge potentials of these new composite electrode materials in a wide range of SCs suitable for future energy-storage devices, however huge data inconsistency remains to be pointed out. In a three-electrode setup, the tiny amount of composite attached to the electrode to be tested imposes large uncertainty on the final outcome. In this review, it is impossible to cover all recent progress, but we collected some important works and presented them in
Table 5 to highlight the different electrode materials and their performance in SCs.
The utilization of carbonaceous materials as electroactive materials has multiple advantages, such as (i) high specific surface area, (ii) low cost, (iii) wide availability, and (iv) mature electrode production technologies. Having all these features, graphene has logically attracted huge research interest in SCs since its discovery. Graphene is a single-layer
sp2-hybridized carbon layer with a honeycomb structure, exhibiting many unique properties, including high carrier mobility, high thermal conductivity, and strong mechanical behavior. However, the valence and conduction bands of graphene are overlapped, which hinders its direct use in electronic applications. To overcome this, it is essential to generate band gaps and tune the activity in graphene. Another challenge for graphene to be used in electronic applications lies in the π–π stacking interactions of the graphitic sheets, which tends to result in their self-aggregation [
108]. To counter this aggregation challenge, the distribution of metal/metal oxide over the graphitic sheets could be a useful strategy, which could also increase the surface area and conductivity of the graphitic sheets [
109].
Among various metal oxides, NiO has been widely investigated for SC applications. Ramesh et al. recently reported the synthesis of NiO/MnO
2@N-doped graphene oxide for SC electrode material [
110,
111]. The composite was nanocrystalline in nature with a large surface area and facilitated ion/electron transport. The composite offered a specific capacitance of 1490 F/g at a current density of 0.5 A/g. The synergistic effect of NiO@MnO
2 oxides over the graphitic sheets was believed to result in the very high capacitance. Xi et al. reported NiO/MnO
2 core–shell nanoflakes over a carbon cloth for flexible SC electrodes [
111], which is essential for further development of flexible and foldable electronic gadgets. They grew NiO on the carbon cloth using a hydrothermal process and then an MnO
2 thin film covered the NiO structure by the self-limiting process. Field emission scanning electron microscopy (FESEM) images (
Figure 4) of the fabricated NiO-coated MnO
2 showed beautiful flower petal morphology that is a signature of high surface area of the developed prototype materials for hybrid SCs [
110,
111]. The NiO nanosheets were grown perpendicularly over the carbon fibers, which led to a highly porous structure beneficial for the diffusion of electrolyte into the electrode [
112].
It was observed that upon further deposition of the MnO
2 layer, the morphology was retained, and the results revealed that the hybrid structure was highly integrated. Such nanoflakes exhibited an aerial specific capacitance of 316.37 mFcm
−2 with a Coulombic efficiency of > 97%. The as-synthesized binder-free electrode possessed superior electrochemical behavior for flexible SCs. Liu et al. reported tunable sulfuration engineered electrodes made from NiO/Ni
3S
2 nanosheets constructed over a porous Ni foam and achieved a specific capacitance of 2153 F/g [
112]. They constructed an asymmetric SC device using the NiO/Ni
3S
2 nanosheets as the positive electrode, activated carbon as the negative electrode, and 3 M KOH as the electrolyte, and the device performance was evaluated by cyclic voltammetry (CV) study [
109,
110,
111]. Liu et al. considered that the redox reactions were the main reason for the good performance, and the CV features of their testing results are shown in
Figure 5.
From
Figure 5, Liu et al. concluded that a linear dependence between the cathodic peak current and the square root of the scan rate presented in the redox reaction at the electrode/electrolyte interface was caused by the diffusion-controlled non-surface processes. They also suggested that the sulfuration treatment played a vital role in the fast redox reactions, thereby increasing the capacitive behavior [
112]. A schematic of the synthesis of electrode material for SCs using NiO/Ni
3S
2 nanosheets over Ni foam is presented in
Figure 6.
From the schematic, it is clear that the morphology of the resulting nanostructures depends on the sulfuration reaction time (8 h, 12 h, and 16 h), as presented in
Figure 6. He et al. synthesized another electrode material for SCs over Ni foam, namely tremella-like NiC
2O
4@NiO core/shell nanostructures, which offered a specific capacitance of 2287 F/g at a current density of 1 A/g [
113]. They demonstrated that such tremella-like morphology (
Figure 7) increased the number of active sites for the redox reactions, which enabled the effective penetration of the electrolyte, shortened the diffusion pathway, and thereby increased the conductivity [
114]. He et al. studied the structural and morphological changes of the fabricated samples using powder X-ray diffraction (XRD) technique (
Figure 7), and a distinct variation in the diffraction patterns was observed, which is in good accordance with the morphological features. To evaluate the electron-hopping process on the surface of materials that is the backbone of charge accumulation and redox reactions, electrochemical analyses were carried out in 2 M KOH solution. All the CV curves depicted the well-defined redox peaks, indicating the pseudocapacitive behavior of the nanocomposites [
113]. In an alkaline solution, the faradaic reaction occurred according to the following equation:
Thus, due to the extra electrons formed, the heterostructure electrode exhibited a high specific capacitance, with a good rate capability and cyclic stability. After 10,000 cycles, the capacitance remained at 95% of the initial value, which is very impressive.
This pioneering work inspired many researchers to further test analogue materials for their applicability in SCs. Within 4 years (2015–2018), a huge number of works on tremella-like core/shell nanostructures using different metals were published, and some highly efficient hybrid materials have been identified for SCs [
111,
112]. However, the selection of an electrolyte for these electrode materials demands further optimization, because a poor combination with an electrolyte could create problems, not only for efficiency but for future commercialization as well [
112,
113].
In this regard, several metal sulfides have attracted special attention due to their easy synthesis [
113,
114]. Among many of them, nanosized NiS is a typical pseudocapacitive electrode material for SCs. Its salient features, such as high redox activity, good capacitive performance, and ease of processability, make it particularly attractive [
114]. Guan et al. reported the synthesis of NiS micro-flowers, with improved surface area and enhanced electron transfer rates, the electrode made of these flowers exhibited high capacitive performance, offering a specific capacitance of 1122.7 F/g at a current density of 1 A/g [
114]. Such a hierarchical structure facilitated easy access of its surface to the electrolyte. The electrochemical activity in 3 M KOH electrolyte showed faradaic reactions according to the following equation:
An asymmetric capacitor, using the micro-flowers as the positive electrode against an activated carbon negative electrode, delivered an energy density of 31 Wh/kg with a power density of 0.9 kW/kg. In addition, the asymmetric capacitor showed capacitance retention of 114.1% at 5 A/g. The morphology of the nanostructured NiS was characterized by SEM and transmission electron microscopy (TEM), and the results are presented in
Figure 8. The flower-like morphology depicts the porous structure and high surface area in the SEM images, while the dark spots in the TEM images show the heterogeneous structure along with a homogeneous coating of NiS [
114].
Owing to its abundance, low cost, and high specific capacitance feature, Mn
3O
4 has also been explored as an electrode material for SC applications. Xiong et al. prepared Mn
3O
4 nanoparticles and incorporated them in rGO films, aiming to develop flexible electrodes [
93]. Their asymmetric SCs showed a volumetric capacitance of 52.5 Fcm
−3 at 0.2 Acm
−3 in a Na
2SO
4 electrolyte [
93,
94]. The intercalation of Mn
3O
4 nanoparticles into the rGO paper improved the conductive behavior of the rGO. Surface morphology studies revealed that the nanoparticles were uniformly distributed between the rGO layers, which resulted in efficient charge transfer and reduced the restacking issues of the rGO sheets [
93]. Meanwhile, the rGO sheets also acted as frameworks to support the Mn
3O
4 nanoparticles and prevented them from dissolution and aggregation (
Figure 9). Such a synergistic effect between the two components led to the formation of an easy and continuous ion-transport network with enhanced rate kinetics and stability [
93].
Liu et al. reported a similar rGO/MnOx@carbon hollow nanosphere (HCNS) core–shell structure for application in SC electrodes [
115]. The method for the preparation of these nanocomposites is depicted in
Figure 9. They have proposed that the Mn
2+ bind with the negatively charged O
2− of the graphene oxide, which leads to a 3D core–shell structure offering good electrochemical behavior with an 88% capacitance retention after 5000 cycles (
Figure 10).
The composite possesses a hollow geometry with a uniform outer shell of 10 nm and inner spherical pores of 150 nm. CV studies were carried out in a two-electrode system in a 6 M KOH electrolyte. The electrode material showed an energy density of 9.38 Wh/kg and a power density of 500 W/kg at a current density of 1 A/g. The material also offered a high rate capability with a specific capacitance of 250 F/g in the two-electrode configuration [
115]. Thus, the new nano-architecture highlights a great potential for core–shell electrode materials in SCs. The surface morphology of all the components used in their study is shown in
Figure 11. The SEM morphology provides clear information regarding the internal structure of these nano- and macro-materials. It is interesting to note that the spherical shape remains in every sample (viz. (a) SiO
2, SiO
2@GO, SiO
2@RGO/MnO
x, and RGO/MnO
x@HCNs).
Another porous nanostructured material for SC application was reported by Beka et al. [
116]. They synthesized a nickel-cobalt sulfide (NiCo
2S
4) core–shell structure on a 3D graphene framework and consequently evaluated its electrochemical performance. In their synthesis, a NiCo
2S
4 nanotube (NCS) acted as the core and Co
xNi
(3−x) S
2 (CNS) nanosheets as the shell. The 3D graphene was first grown over the Ni foam using a chemical vapor deposition process, named graphene nanoflake (GNF), and then the NCS/CNS was grown on the 3D graphene using a hydrothermal process. The high mechanical stability of 3D graphene provided excellent support for the entire system, and the high conductivity of the graphene network offered superb transport channels between the collector and active material [
116]. The uniform porous forest became a 3D architecture with more surface-active sites after the core/shell formation, thus leading to increased charge storage [
116].
Figure 12 shows the surface morphology of the reported electrode materials.
The graphene layer sandwiched between the NCS/CNS core–shell and the nickel foam current collector aided the superb electron transport and led to an aerial capacitance of 15.6 F cm
−2 at a current density of 10 mA cm
−2, with cyclic stability of 93% after 5000 cycles and a rate capability of 74.36% [
116]. Zhou et al. reported a new hybrid material with layered porous structure as SC electrodes [
110]. They studied the performance of a new series of layered barium transition-metal fluorides, BaMF4 (M = Mn, Co, and Ni). In the layered structure, spaces existed between layers, leading to the formation of numerous interfaces [
110]. The interlayer spaces acted as a reservoir for the anions, which could be driven in and out depending on the externally applied electric field or inner built electric field.
Figure 13 shows the arrangement of the metal atoms in the lattice structure. In the density-functional theory (DFT) investigation it was found that among the three studied electrode systems, M ¼ Co showed the largest capacitance, best conductivity, and cycle stability. Considering the very early stage of using BaMF4 as electrodes, we believe that there is enough space in the near future to greatly improve their SC performance [
110,
117].
Zhou et al. further compared the theoretical results with experimental results. The morphology of the studied nanomaterials ((a) BaMnF4, (b) BaCoF
4, and (c) BaNiF
4) is shown in
Figure 14. Interestingly, the shapes of the crystals appearing in the SEM micrographs were amazingly similar to the results shown in
Figure 13. The SEM morphology (
Figure 14) of the bulk materials (BaCoF4 and BaNiF4) was not suitable for application as SC electrode materials, owing to very low surface area and lack of porosity. These SEM images agreed well to the theoretical results, as obtained by Zhou et al. [
110]. However, its exfoliation under suitable conditions produced an ideal nanostructure that was suitable for the SC applications.
The surface morphology analyses, as shown in
Figure 14, revealed that a layered structure with a stratified structure was formed, due to the anisotropic crystal growth under hydrothermal conditions, which resulted in a quasi-2D crystalline structure. The puckered sheets can provide free space with the increased surface area for fast ion diffusion, which benefits the electrode performance. The valence-variable metal ions led to improved faradaic redox reactions at the electrode interface, which contributed to the electrochemical performance of the electrode material. CV and electrochemical impedance spectroscopy (EIS) studies in a three-electrode system with 6 M KOH electrolyte depicted the specific capacity and specific capacitance of BaMF
4 by varying the M centers with Mn, Co, and Ni. The EIS study (
Figure 15) also revealed the equivalent series resistance of the respective systems.
Table 6 tabulates the data corresponding to the different metal centers [
110,
117].
Layered double hydroxides (LDHs) have been extensively cultivated as a potential pseudocapacitive material, since their high specific capacitance, good redox reversibility, and excellent ion-exchange property [
17]. Compared with binary-component LDH, ternary-component LDH has much better electrochemical activity because of an increased number of active sites after incorporation of the third metal ion. Such a ternary-component LDH has been studied by Wang et al., who reported the synthesis of CoNiFe-LDH/carbon nanofibers (CNFs) [
17]. The composite CoNiFe-LDH/CNFs displayed a high specific capacitance of 1203 F/g at a current density of 1 A/g and an excellent long-term cyclic stability of 94.4% after 1000 cycles. Wang et al. also constructed an asymmetric supercapacitor with CoNiFe-LDH/CNFs as the positive electrode and activated carbon as the negative electrode. Their device offered a specific capacitance of 84.9 F/g at a current density of 1 A/g and an energy density of 30.2 Wh/kg [
17]. The SEM images of the original CNFs in
Figure 16 show the entangled-network structure with uniform dispersion. The SEM image of CoNiFe-LDH also presents the formation of irregular nanosheets. After the incorporation of CNFs, the aggregated structure in
Figure 16b is somewhat reduced, as shown in
Figure 16c,d, and such a structure was beneficial for faster ion diffusion and electronic transportation. Using elemental mapping, Wang et al. also proved the presence of three metal centers in the composite. The further detailed structure of the hollow tubular CNFs was also unveiled using TEM images (
Figure 17) [
17]. Based on these HRTEM and SEM images, Wang et al. claimed that they fabricated the nanocomposite with high surface areas, which contributed to the excellent performance [
17].
Indeed, by incorporating CNFs into the double-layer hydroxide, the rate capability and specific capacitance of the composite were significantly improved, consistent with their CV results, as shown in
Figure 18.
The FESEM study of the synthesized composite revealed a network-like intertwined structure, which can be effective for enhancing the surface area, thereby leading to easy accessibility of the electrolyte ions. BET surface-area analysis has also demonstrated that FcGA exhibited a maximum surface area of 231 m
2g
−1, which could also influence the electrochemical performance. The electrochemical analysis was carried out in 1 M NEt
4BF
4-acetonitrile solution as depicted in
Figure 19, and the electrodes gave a high specific capacitance of 960 F/g and an energy density of 76.44 Wh/kg at a current density of 1 A/g.
In recent years, double-hydroxide-based SCs have attracted significant attention, and several outstanding works on the subject have been published [
118,
119,
120]. In this regard, by advancing the fabrication of graphene-based composites as electrode materials for SC applications, our research group achieved an Mg-Al LDH using rGO [
121]. Utilizing a one-pot synthesis approach, we first produced sandwich-like Mg/Al LDH anchored with rGO to form the composite. Hatui et al. explained that the exceptional efficiency of these electrode materials was due to the unique manner of electron transfer from metallic layers to rGO. The process of electron transfer from one phase to the other according to Hatui and coworkers was schematically presented in
Figure 20 [
121].
Hatui et al. used a variety of techniques, including Fourier-transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), and XRD analysis along with FESEM, TEM, and atomic-force microscopy (AFM), to verify the sandwiched structure [
121]. It has been found that the special sandwiched morphology of the newly developed rGO-based Mg/Al LDH played a very crucial role in achieving maximum performance [
121]. Selected FESEM images and energy-dispersive X-ray (EDX) graphs of such samples are exhibited in
Figure 21.
Hatui et al. also carried out electrochemical analyses and characterizations of their composites in 1 M aqueous KOH, which gave rise to a specific capacitance of 1334 F/g at a current density of 1 A/g in a three-electrode system [
121]. To avoid errors, they also tested the efficiency of developed materials with a two-electrode cell system and recorded a specific capacitance of 1092.5 F/g in 1 M TEABF4 (in acetonitrile solution) as the electrolyte at a current density of 2 A/g. They further reported cyclic stability of approximately 87% retention of specific capacitance after 10,000 consecutive charge–discharge cycles at a steady current density of 5 A/g for the two-electrode organic electrolyte system. The energy density of the nanocomposite also shows a high value of 388.26 Wh/kg at a current density of 2 A/g and a power density of 3198.48 W/kg in the two-electrode organic electrolyte system. The importance advance of this DLH material over others is mainly down to the very simple, one-pot solvothermal technique, which is the cheapest and most facile route for the fabrication of sandwich-like RGO@ MgAl LDH nanocomposites. Moreover, this method also offered a simultaneous reduction of GO to rGO, and therefore the growth of MgAl LDH could greatly enhance both the conductivity of the entire system and reduce the internal resistance, which, in turn, may improve the specific capacitance due to the very high surface area of the nanocomposites [
121]. Thus the discussions of various studies carried out by different research groups in recent years confirm the wide applicability of SCs in future energy-storage devices. The SCs can supply quick bursts of power for consumer electronics and vehicles, thus help to reduce the need for non-renewable energy sources, eventually contributing to reduce their depletion.
We appreciate that the work reported by Hatui et al. adopted a very easy and facile route for the synthesis of LDH-based graphene interlinked nanocomposites for prototypical SC applications. The proposed mechanism for the electron hopping from the LDH to rGO in the electrode materials for the SC applications was diagrammatically presented in
Figure 20. It is interesting to note that the newly developed LDH using rGO showed a much greater efficiency than those reported previously, although Hatui et al. adopted a very simple and green method for the materials fabrication [
122,
123,
124,
125,
126]. Some recently developed LDH works are summarized in
Table 7 for further reading.
Amrita and coworkers developed a very smart rGO-based nanomaterial, involving the fabrication of hierarchical Zn-doped SnO
2 nano-urchins decorated on the rGO nanosheets (
Figure 22) as electrode materials for SCs [
130]. The composite electrode offered a specific capacitance of 635 F/g at a current density of 1 A/g and had high cyclic stability up to 5000 cycles with a capacitance retention of 78.4% [
130]. They believed that the excellent energy storage capacity originated from the Zn-doped SnO
2 nanospheres in the presence of rGO, which tailored the morphology and the electrical properties [
24]. They explained that Zn
2+ doping in the SnO
2 nanospheres prevented Sn clustering, thereby reducing the particle size, which led to the formation of urchin-like nanostructures. These urchins with a high surface area and short transport paths can offer high capacitive performance through assembly with rGO nanosheets. Amrita and coworkers used XPS and XRD techniques to confirm the presence of SnO
2 crystal planes and the successful Zn
2+ doping. The incorporation of the rGO nanosheets augmented the Coulombic efficiency, specific capacitance, and cyclic performance. The enhancement of the capacitive behavior was attributed to the synergistic effects of the pseudocapacitance and double-layer capacitance.
Amrita et al. evaluated the surface morphology of their nanocomposites using FESEM, and the results were shown in
Figure 23a, in which the SnO
2 nanospheres exhibited a very uniform spherical structure [
130]. Upon doping the SnO
2 with Zn
2+, a clear spike-like morphology was observed, which resembles a sea urchin structure (inset of
Figure 23a–f). Furthermore, with the addition of CNTs into SnO
2 and Zn-doped SnO
2, as named as SnO2@CNT and ZnSnO
2@CNT for comparison, shown in
Figure 23c,d, the nanospheres and nano-urchins were found nicely intertwined within the CNTs. Upon the addition of CNTs, the nanospheres and nano-urchins were well separated from each other, having condensed size. This configuration resulted in a very high surface area and uniform distribution. By incorporating rGO further with these nanomaterials, as SnO
2@G and ZnSnO
2@G, the nanospheres were also found wrapped by the rGO nanosheets, as revealed in
Figure 23e,f. The yellow surrounded area in
Figure 23e depicts the presence of rGO sheets inside which the SnO
2 nanospheres are integrated.
Figure 23f also shows the uniform and good distribution of the nano-urchins over the rGO. The yellow area indicates the presence of rGO nanosheets within the nano-urchin structures. Further magnified FESEM micrograph of the ZnSnO
2@G illustrates the fine dissemination of nano-urchins over rGO, as arrowed in
Figure 23f. These vivid surface morphological studies on these nanocomposites have revealed the effective assembly of the nano-urchins over the rGO or CNTs, as well as the effect of Zn
2+ doping.
Prior to Amrita et al., Li and their coworker have developed a facile approach to fabricate three-dimensional ZnO-rGO-ZnO sandwich-structures by incorporating ZnO powder into the reaction of graphitic oxide, followed by heating until the proper reduction occurred [
100]. In this process, fine ZnO nanorod arrays with the size of 20–40 nm grew on both surfaces of rGO nanosheets unswervingly. Compared with plain rGO, the as-synthesized ZnO/rGO/ZnO nanocomposites displayed higher specific surface areas [
100]. The electrochemical behavior of ZnO/rGO/ZnO nanocomposites electrodes obtained the highest specific capacitance of 275 F/g at a scan rate of 5 mVs
−1 in 1.0 M Na
2SO
4 electrolyte, by chronopotentiometry. However, according to authors, the repeatability is essential to be double-checked and further study is needed.
They also showed that the as-fabricated hybrid ZnO/rGO/ZnO sandwich-structures exhibited an excellent rate capability and outstanding long-term cycling stability, as compared with the pure rGO and pristine rGO. This excellent work of Li and the coworker established that this ZnO/rGO/ZnO nanostructure is an auspicious candidate as electrode material for high-performance SCs [
100].
To evaluate the electrochemical performance of these materials as a working electrode, Amrita et al. conducted CV and galvanostatic charging–discharging analyses in an aqueous 1 M KCl electrode, in a potential window of 0–0.8 V using a three-electrode system.
Figure 24 shows the CV curves of all nanocomposites at a scan rate of 10 mVs
−1. The CV curves all depict a quasi-rectangular nature without any obvious peak, indicating the typical behavior of the EDLCs. The symmetric quasi-rectangular forms and an increased current density indicate the fast and reversible faradaic reactions and perfect capacitive behavior. A slower voltage scan at 10 mVs
−1 was carried out to reduce the flux at the electrode surface compared with higher scan rates. This can be attributed to the fact that as the current is directly proportional to the flux, the magnitude of the current should be high at faster scan rates and low at slower scan rates. The results of the electrochemical analyses for these electrode materials are presented in
Figure 25a,b [
130]. In addition, the cyclic stability of their materials was tested up the 5000 cycles, and the results are presented in terms of Nyquist-plot specific capacitance retention parameters (
Figure 24).
In addition to the above SnO
2-based electrode materials, many other types of materials, particularly MoS
2, an analogue of 2D graphene and its derivatives, have been intensively investigated by different groups [
130,
131,
132,
133,
134,
135,
136,
137,
138]. These emerging materials have shown great potentials for practical applications. The layered MoS
2 nanostructures can contribute to the double-layer capacitance from their interlayer charge storage, in addition to their intralayer charge-storage, thus becoming an ideal candidate as SC electrode materials. Similar to the much-studied RuO
2, MoS
2 may also provide an added capacitance from the faradaic reaction at the Mo metal center, owing to its multiple and tunable oxidation states varying from 2
+ to 6
+ [
133,
134,
135,
136,
137]. Furthermore, the 2D layered nanostructure of MoS
2 provides excellent electron hopping, and greater ionic conductivity, compared with its corresponding oxides for the same purposes.
Very recently, Nandi et al. reported a direct growth of MoS
2 on a 2D stainless-steel foil surface and on a 3D Ni foam by a plasma-enhanced atomic-layer-deposition (ALD) technique. They then utilized these structures directly as an electrode for SC without any further modifications [
138]. They additionally reported the application of molybdenum hexacarbonyl [Mo(CO)
6], as a prototype emergent ancestor for low-temperature ALD fabrication, to deposit MoS
2. To obtain molybdenum hexacarbonyl, a halide precursor (MoCl
5) and H
2S as a chemical reactant were deposited on the MoS
2 films at an elevated temperature of 300 °C [
138]. Therefore, Nandi et al. adopted hexacarbonyl precursor and H
2S plasma as the chemical reactant. After a very comprehensive investigation of the properties of these nanomaterials and of their growth on the 3D Ni foam, the electrochemical studies were carried out, to realize the potential of plasma-enhanced atomic layer deposition (PEALD) MoS
2 as an electrode in asymmetric SCs. The efficiency of the materials developed in terms of capacitance retention was reported, as shown in
Figure 26. The newly developed method looks unique and simple for the synthesis of high-performance hybrid SC electrode materials.
It is difficult to review all published work related to MoS
2 for SC applications, therefore we selectively summarize some excellent investigations on MoS
2 based electrode materials, in addition to other graphene-based and hybrid composites, as enlisted in
Table 8.