Tetracycline Photocatalytic Degradation under CdS Treatment
Abstract
:1. Introduction
2. Experimental
2.1. Materials, Reagents and Instrumentation
2.2. Synthesis of CdS Hydrophilic Nanoparticles
2.3. CdS Nanoparticles Characterization
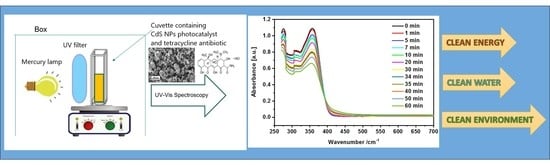
2.4. Photocatalysis Study
3. Results and Discussion
3.1. Morphology, Size, and Zeta-Potential Analysis.
3.2. Structural Studies
3.3. Optical Absorption Studies
3.4. Basic Photoelectrochemical Studies
3.5. Photocatalytic Activity of CdS Nanoparticles
3.5.1. Photocatalytic Discoloration/Decomposition of a Model Compound: Methylene Blue (MB) Dye
3.5.2. Photocatalytic Activity of CdS Towards Tetracycline (TC)
3.5.3. Mechanism of Photocatalytic Decomposition of MB and TC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoon, S.J.; Hong, S.; Kim, S.; Lee, J.; Kim, T.; Kim, B.; Kwon, B.-O.; Zhou, Y.; Shi, B.; Liud, P.; et al. Large-scale monitoring and ecological risk assessment of persistent toxic substances in riverine, estuarine, and coastal sediments of the Yellow and Bohai seas. Environ. Int. 2020, 137, 105517–105529. [Google Scholar] [CrossRef] [PubMed]
- Iduk, U.; Samson, N. Effects and Solutions of Marine Pollution from Ships in Nigerian Waterways. Int. J. Sci. Eng. Res. 2015, 9, 81–90. [Google Scholar]
- Shahidul Islam, M.S.; Tanaka, M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: A review and synthesis. Mar. Pollut. Bull. 2004, 48, 624–649. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Dahrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. 6), 907–938. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams. 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [Green Version]
- Letteri, T.; Napierska, D.; Loos, R.; Martinov, D.; Sanseverino, I. Review of the 1st Watch List Under the Water Framework Directive and Recommendations for the 2nd Watch List; Publication Office of the European Union: Luxembourg, 2018.
- Jeong, Y.; Kim, Y.; Jin, Y.; Hong, S.; Park, C. Comparison of filtration and treatment performance between polymeric and ceramic membranes in anaerobic membrane bioreactor treatment of domestic wastewater. Sep. Purif. Technol. 1999, 199, 182–188. [Google Scholar] [CrossRef]
- Agunbiade, M.O.; Van Heerden, E.; Pohl, C.H.; Ashafa, A.T. Flocculation performance of a bioflocculation by Arthrobacter humicola in sewage waste treatment. BMC Biotechnol. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Yu, W.; Xu, L.; Graham, N.; Qu, J. Pre-treatment for ultrafiltration: Effect of pre-chlorination on membrane fouling. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Strizhak, P.A. Coagulation and splitting of droplets of coal-water slurry containing petrochemicals and their effect on ignition characteristics. Appl. Therm. Eng. 2017, 116, 266–277. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Martinez, A.; Patureau, D.; Delgenès, J.-P.; Carrère, H. Removal of polycyclic aromatic hydrocarbons (PAH) during anaerobic digestion with recirculation of ozonated digested sludge. J. Hazard. Mater. 2009, 162, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.J.; Bai, J.; Liu, K.K.; Sun, H.M.; Zhao, Y.G. Occurrence and removal of polycyclic aromatic hydrocarbons in the wastewater treatment process. Ecotoxicol. Environ. Saf. 2012, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Smol, M.; Włodarczyk-Makuła, M. The Effectiveness in the Removal of PAHs from Aqueous Solutions in Physical and Chemical Processes: A Review. Polycycl. Aromat. Compd. 2007, 37, 292–313. [Google Scholar] [CrossRef]
- Singh, P.; Ojha, A.; Borthakur, A.; Singh, R.; Lahiry, D.; Tiwary, D.; Mishra, P.K. Emerging trends in photodegradation of petrochemical wastes: A review. Environ. Sci. Pollut. Res. 2016, 23, 22340–22364. [Google Scholar] [CrossRef]
- Hsu, Y.-Y.; Hsiung, T.-L.; Wang, H.P.; Fukushima, Y.; Wei, Y.-L.; Chang, J.-E. Photocatalytic degradation of spill oils on TiO2 nanotube thin films. Mar. Pollut. Bull. 2008, 57, 873–876. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and inactivation of tetracycline by TiO2 photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Zhu, X.-D.; Wang, Y.-J.; Sun, R.-J.; Zhou, D.-M. Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 2013, 92, 925–932. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Mashayekhi, M.; Naderi, M.; Boczkaj, G.; Jorfi, S.; Safari, M. Sonocatalytic degradation of tetracycline antibiotic using zinc oxide nanostructures loaded on nano-cellulose from waste straw as nanosonocatalyst. Ultrason. Sonochem. 2019, 55, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, X.; Yang, J.; Liu, G.; Han, J.; Li, C. Dual cocatalysts loaded type I CdS/ZnS core/shell nanocrystals as effective and stable photocatalysts for H2 evolution. J. Phys. Chem. C 2013, 117, 11584–11591. [Google Scholar] [CrossRef]
- Khan, Z.R.; Zulfequar, M.; Khan, M.S. Chemical synthesis of CdS nanoparticles and their optical and dielectric studies. J. Mater. Sci. 2011, 46, 5412–5416. [Google Scholar] [CrossRef]
- Schiavello, M. Photocatalysis and Environment; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Serpone, M.; Pelizzeti, E. Photocatalysis, Fundamentals and Applications; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Ollis, D.F.; Al-Ekabi, H. Photocatalytic Purification, and Treatment of Water and Air; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Legrini, O.; Oliveros, E.; Braun, A. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Herrman, J.M.; Guillard, C.; Pichat, P. Heterogeneous Photocatalysis: An emerging technology for water treatment. Catal. Today 1993, 17, 7–20. [Google Scholar] [CrossRef]
- Bahnemann, D.W.; Cunningham, J.; Fox, M.A.; Pelizzetti, E.; Pichat, P.; Serpone, N.; Helz, G.R.; Zepp, R.G.; Crosby, D.G. Aquatic and Surface Photochemistry; Lewis: Boca Raton, FL, USA, 1994. [Google Scholar]
- Blake, D.M. Bibliography of Work on Photocatalytic Removal of Hazardous Componds from Water and Air; National Renewable Energy Laboratory: Golden, CO, USA, 1997.
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Hoffman, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Mills, A.; LeHunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.D. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energ. 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic Materials for Enviornmental Applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B. Photocatalysis A to Z: What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C 2010, 11, 157–178. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, R.; Waseem, A.; Iqbal, A.; Shah, Z.H. CdS nanocapsules and nanospheres as efficient solar light-driven photocatalysts for degradation of Congo red dye. Inorg. Chem. Commun. 2016, 72, 33–41. [Google Scholar] [CrossRef]
- Atabaev, T.S. Facile hydrothermal synthesis of flower-like hematite microstructure with high photocatalytic properties. J. Adv. Ceram. 2015, 4, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Biao, L.; Dan, L.; Lei, M.; Sung, Y.I. One-pot synthesis of Cu2O octahedron particles and their catalytic application. Bull. Korean Chem. Soc. 2017, 38, 499–502. [Google Scholar]
- Rao, C.N.R.; Vivekchand, S.R.C.; Biswas, K.; Govindaraj, A. Synthesis of inorganic nanomaterials. Dalton Trans. 2007, 34, 3728–3749. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.-J.; Jiang, L.-P.; Liu, C.; Zhu, J.-M.; Zhu, J.-J. General sacrificial template method for the synthesis of cadmium chalcogenide hollow structures. Inorg. Chem. 2007, 46, 5673–5677. [Google Scholar] [CrossRef]
- Pham, L.Q.; Van, T.-K.; Cha, H.G.; Kang, Y.S. Controlling crystal growth orientation and crystallinity of cadmium sulfide nanocrystals in aqueous phase by using cationic surfactant. Cryst. Eng. Comm. 2012, 14, 7888–7890. [Google Scholar] [CrossRef]
- Wu, Y.; Wadia, C.; Ma, W.; Sadtler, B.; Alivisatos, A.P. Synthesis and photovoltaic application of copper [I] sulfide nanocrystals. Nano Lett. 2008, 8, 2551–2555. [Google Scholar] [CrossRef]
- Guo, Q.; Ford, G.M.; Yang, W.-C.; Walker, B.C.; Stach, E.A.; Hillhouse, H.W.; Agrawal, R. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals. J. Am. Chem. Soc. 2010, 132, 17384–17386. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, J.; Yang, L.; Zhang, J.; Jiang, K.; Li, W.; Wang, L.; Jiang, L. Facile additive-free solvothermal synthesis of cadmium sulfide flower-like three dimensional assemblies with unique optical properties and photocatalytic activity. Cryst. Eng. Comm. 2011, 13, 5045–5048. [Google Scholar] [CrossRef]
- Arora, M.K.; Sahu, N.; Upadhyay, S.N.; Sinha, A.S.K. Activity of cadmium sulfide photocatalysts for hydrogen production from water: Role of support. Ind. Eng. Chem. Res. 1999, 38, 2659–2665. [Google Scholar] [CrossRef]
- Vázquez, A.; Hernández-Uresti, D.B.; Obregón, S. Electrophoretic deposition of CdS coatings and their photocatalytic activities in the degradation of tetracycline antibiotic. Appl. Surf. Sci. 2016, 386, 412–417. [Google Scholar] [CrossRef]
- Prashant, J.; Ginimuge, R.; Jyothi, S.D. Methylene Blue: Revisited. Anaesthesiol. Clin. Pharmacol. 2010, 26, 517–520. [Google Scholar]
- Farombi, E.O.; Ugwuezunmba, M.C.; Ezenwadu, T.T.; Oyeyemi, M.O.; Ekor, M. Tetracycline-induced reproductive toxicity in male rats: effects of vitamin C and N-acetylcysteine. Exp. Toxicol. Pathol. 2008, 60, 77–85. [Google Scholar] [CrossRef]
- He, X.; Nguyen, V.; Jiang, Z.; Wang, D.; Zhu, Z.; Wang, W.-N. Highly-Oriented One-Dimensional MOF-Semiconductor Nanoarrays for Efficient Photodegradation of Antibiotics. Catal. Sci. Technol. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Bera, R.; Kundu, S.; Patra, A. 2D Hybrid Nanostructure of Reduced Graphene Oxide−CdS Nanosheet for Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 13251–13259. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Wu, W.; Wang, S.; Qiang, L. Highly efficient toward tetracycline under simulated solar-light by Ag+-CDs-Bi2WO6: Synergistic effects of silver ions and carbon dots. Appl. Catal. B Environ. 2016, 192, 277–285. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tangb, L.; Zhoub, Y.; Zoub, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Safari, G.H.; Hoseini, M.; Seyedsalehi, M.; Kamani, H.; Jaafari, J.; Mahvi, A.H. Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int. J. Environ. Sci. Technol. 2015, 12, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-S.; Tsai, R.-W.; Chen, Y.-S.; Huang, K.-L. Electrochemical Degradation of Tetracycline on BDD in Aqueous Solutions. Int. J. Electrochem. Sci. 2014, 9, 8422–8434. [Google Scholar]
- Jin, C.; Li, W.; Chen, Y.; Li, R.; Huo, J.; He, Q.; Wang, Y. Efficient Photocatalytic Degradation and Adsorption of Tetracycline over Type-II Heterojunctions Consisting of ZnO Nanorods and K-Doped Exfoliated g-C3N4 Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 2860–2873. [Google Scholar] [CrossRef]
- Li, W.; Ding, H.; Ji, H.; Dai, W.; Guo, J.; Du, G. Photocatalytic Degradation of Tetracycline Hydrochloride via a CdS-TiO2 Heterostructure Composite under Visible Light Irradiation. Nanomaterials 2018, 8, 415. [Google Scholar] [CrossRef] [Green Version]
- Nasseh, N.; Panahi, A.H.; Esmati, M.; Daglioglud, N.; Asadi, A.; Rajati, H.; Khodadoost, F. Enhanced photocatalytic degradation of tetracycline from aqueous solution by a novel magnetically separable FeNi3/SiO2/ZnO nano-composite under simulated sunlight: Efficiency, stability, and kinetic studies. J. Mol. Liq. 2020, 301, 112434–112442. [Google Scholar] [CrossRef]
- Tang, T.; Liu, X.; Ma, C.; Zhou, M.; Huo, P.; Yu, L.; Pan, J.; Shi, W.; Yan, Y. Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide–CdS/ZnS heterostructure photocatalysts. New J. Chem. 2015, 7, 1–37. [Google Scholar] [CrossRef]
- Suresh, S. Studies on the dielectric properties of CdS nanoparticles. Appl. Nanosci. 2014, 4, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Kundu, J.; Pradhan, D. Controlled synthesis and catalytic activity of copper sulfide nanostructured assemblies with different morphologies. Appl. Mater. Interfaces 2014, 6, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Khilari, S.; Pradhan, D. Shape-dependent photocatalytic activity of hydrothermally synthesized cadmium sulfide nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 9669–9680. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.; Weinstein, M.; Wolff, G.A. Ultraviolet reflection spectrum of cubic CdS. Phys. Rev. 1965, 140, 633–637. [Google Scholar] [CrossRef]
- Kapoor, S.; Ahmad, H.; Julien, C.M.; Islam, S.S. Improved ion-diffusion assisted uniform growth of 1D CdS nanostructures for enhanced optical and energy storage properties. Appl. Surf. Sci. 2020, 512, 145654–145665. [Google Scholar] [CrossRef]
- Rajbongshi, H.; Kalita, D. Morphology-Dependent Photocatalytic Degradation of Organic Pollutant and Antibacterial Activity with CdS Nanostructures. J. Nanosci. Nanotechnol. 2020, 20, 5885–5895. [Google Scholar] [CrossRef]
- Han, G.; Wang, L.; Pei, C.; Shi, R.; Liu, B.; Zhao, H.; Yang, H.; Liu, S. Size-dependent optical properties and enhanced visible light photocatalytic activity of wurtzite CdSe hexagonal nanoflakes with dominant {001} facets. J. Alloys Compd. 2014, 610, 62–68. [Google Scholar] [CrossRef]
- Bergmane, K.; O’konski, T. A spectroscopic study of methylene blue, dimer, and complexes with montmorillonite. J. Phys. Chem. 1963, 67, 2169–2177. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 2017, 7, 23699–23708. [Google Scholar] [CrossRef] [Green Version]
- Reddy, C.V.; Shim, J.; Cho, M. Synthesis, structural, optical and photocatalytic properties of CdS/ZnS core/shell nanoparticles. J. Phys. Chem. Solids 2017, 103, 209–217. [Google Scholar] [CrossRef]
- Khurana, C.; Vala, A.K.; Andhariya, N.; Pandey, O.P.; Chudasama, B. Influence of antibiotic adsorption on biocidal activities of silver nanoparticles. IET Nanobiotechnol. 2016, 10, 69–74. [Google Scholar] [CrossRef]
- Cao, H.-L.; Cai, F.-Y.; Yu, K.; Zhang, Y.-Q.; Lü, J.; Cao, R. Photocatalytic Degradation of Tetracycline Antibiotics over CdS/Nitrogen-Doped-Carbon Composites Derived from in Situ Carbonization of Metal-Organic-Frameworks. ACS Sustain. Chem. Eng. 2019, 7, 10847–10854. [Google Scholar] [CrossRef]
- López-Peñalver, J.J.; Sánchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of tetracyclines in aqueous solution by using UV and UV/H2O2 oxidation processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Meissner, D.; Memming, R.; Kastening, B. Photoelectrochemistry of cadmium sulfide. 1. reanalysis of photocorrosion and flat-band potential. J. Phys. Chem. 1988, 92, 3476–3483. [Google Scholar] [CrossRef]
- Dewitt, R.; Mesmaeker, A.K.D. Capacitance characteristics of the polycrystalline CdS/NaOH and CdS/cysteine interfaces. J. Electrochem. Soc. 1983, 130, 1995–1998. [Google Scholar] [CrossRef]
- Minoura, H.; Tsuiki, M. Anodic reactions of several reducing agents on illuminated cadmium sulfide electrode. Electrochim. Acta 1978, 23, 1377–1382. [Google Scholar] [CrossRef]
- Li, S.; Hu, J. Photolytic and photocatalytic degradation of tetracycline: Effect of humic acid on degradation kinetics and mechanisms. J. Hazard. Mater. 2016, 318, 134–144. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction process. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef]
- Boonserm, A.; Chaiyaput, K.; Varinrumpai, S. Photoelectrochemical response and corrosion behavior of CdS/TiO2 nanocomposite films in an aerated 0.5 M NaCl solution. Appl. Surf. Sci. 2017, 419, 933–941. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, X.; Yu, L.; Wang, Y.; Ning, J.; Xu, S.; Lou, X.W. Carbon-coated CdS petalous nanostructres with enhanced photostability and photocatalytic activity. Angew. Chem. Int. Ed. Engl. 2013, 52, 5636–5639. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.G.; Kierulf-Vieira, W.; Łęcki, T.; Zarębska, K.; Widera-Kalinowska, J.; Skompska, M. Synthesis and application of N-doped TiO2/CdS/poly(1,8-diaminocarbazole) composite for photocatalytic degradation of 4-chlorophenol under visible light. Electrochim. Acta 2019, 314, 73–80. [Google Scholar] [CrossRef]
- Maranowski, B.; Dulovic, S.; Casto, S.; Strawski, M.; Widera-Kalinowska, J.; Szklarczyk, M. Preparation and Characterization of CdSe/POMA Photoactive Composites Electrochemically Grown on HOPG Surfaces. J. Electroanal. Chem. 2020. [Google Scholar] [CrossRef]














© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagamine, M.; Osial, M.; Jackowska, K.; Krysinski, P.; Widera-Kalinowska, J. Tetracycline Photocatalytic Degradation under CdS Treatment. J. Mar. Sci. Eng. 2020, 8, 483. https://doi.org/10.3390/jmse8070483
Nagamine M, Osial M, Jackowska K, Krysinski P, Widera-Kalinowska J. Tetracycline Photocatalytic Degradation under CdS Treatment. Journal of Marine Science and Engineering. 2020; 8(7):483. https://doi.org/10.3390/jmse8070483
Chicago/Turabian StyleNagamine, Momoka, Magdalena Osial, Krystyna Jackowska, Pawel Krysinski, and Justyna Widera-Kalinowska. 2020. "Tetracycline Photocatalytic Degradation under CdS Treatment" Journal of Marine Science and Engineering 8, no. 7: 483. https://doi.org/10.3390/jmse8070483