Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp.
Abstract
:1. Introduction
2. Materials and Methods
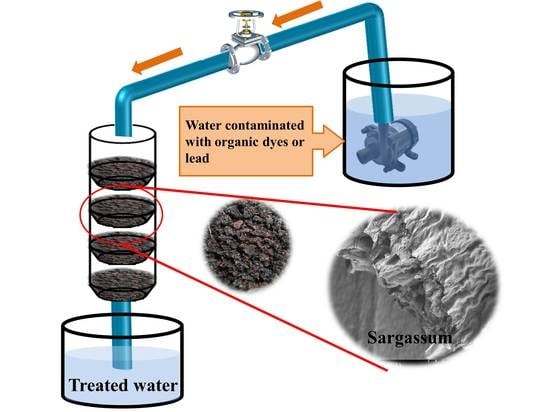
2.1. Filter System based on Sargassum spp. (Sspp)
2.2. Materials
2.2.1. Chemical Treatment of the Sargassum spp. (Sspp)
2.2.2. Characterization of the Sargassum spp. (Sspp).
2.2.3. Evaluation of Variables in the Filtration System
2.3. Analysis of the Samples Obtained by Biosorption
3. Results
3.1. Characterization of Sargassum spp. by Scanning Electron Microscopy (SEM) and FTIR
3.2. Effect of Chemical Treatment of Sargassum spp.
3.3. Amount of Sargassum spp. in Filters
3.4. Mechanically Treated Sargassum spp.
3.5. Evaluation of Filter Diameter
3.6. Evaluation of Filter Layers
3.7. Evaluation of Water Flow Rate
3.8. Evaluation of Sargassum spp. Humidity in the Filters
3.9. Removal of Methyl Orange and Methyl Red
3.10. Removal of Pb Ions
3.11. Comparison of Substance Removal using Sargassum spp.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Liu, S.; Pan, G.; Yang, H.; Cai, Z.; Zhu, F.; Ouyang, G. Determination and elimination of hazardous pollutants by exploitation of a Prussian blue nanoparticles-graphene oxide composite. Anal. Chim. Acta. 2019, 1054, 17–25. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Ferdowsi, M.; Ramirez, A.A.; Jones, J.P.; Heitz, M. Elimination of mass transfer and kinetic limited organic pollutants in biofilters: A review. Int. Biodeterior. Biodegrad. 2017, 119, 336–348. [Google Scholar] [CrossRef]
- Abd El-Rahim, W.M.; Moawad, H.; Azeiz, A.Z.A.; Sadowsky, M.J. Optimization of conditions for decolorization of azo-based textile dyes by multiple fungal species. J. Biotechnol. 2017, 260, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; Mrabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Muniyasamy, A.; Sivaporul, G.; Gopinath, A.; Lakshmanan, R.; Altaee, A.; Achary, A.; Chellam, P.V. Process development for the degradation of textile azo dyes (mono-, di-, poly-) by advanced oxidation process-Ozonation: Experimental & partial derivative modelling approach. J. Environ. Manage. 2020, 265, 110397. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; De Vito, S.C. Predicting azo dye toxicity. Crit. Rev. Env. Sci. Technol. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ. Pollut. 2020, 257, 113514. [Google Scholar] [CrossRef] [PubMed]
- Đurđić, K.I.; Ostafe, R.; Đelmaš, A.Đ.; Popović, N.; Schillberg, S.; Fischer, R.; Prodanović, R. Saturation mutagenesis to improve the degradation of azo dyes by versatile peroxidase and application in form of VP-coated yeast cell walls. Enzym. Microb. Technol. 2020, 136, 109509. [Google Scholar] [CrossRef] [PubMed]
- Semião, M.A.; Haminiuk, C.W.I.; Maciel, G.M. Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye Reactive Blue 160. J. Environ. Chem. Eng. 2020, 8, 103617. [Google Scholar] [CrossRef]
- Jadhav, J.; Parshetti, G.; Kalme, S.; Govindwar, S.P. Decolourization of azo dye methyl red by Saccharomyces cerevisiae MTCC 463. Chemosphere 2007, 68, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Rożek, P.; Król, M.; Mozgawa, W. Lightweight geopolymer-expanded glass composites for removal of methylene blue from aqueous solutions. Ceram. Int. 2020, 46, 19785–19791. [Google Scholar] [CrossRef]
- Beh, J.H.; Lim, T.H.; Lew, J.H.; Lai, J.C. Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal. Int. J. Biol. Macromol. 2020, 160, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Geed, S.R.; Samal, K.; Tagade, A. Development of adsorption-biodegradation hybrid process for removal of methylene blue from wastewater. J. Environ. Chem. Eng. 2019, 7, 103439. [Google Scholar] [CrossRef]
- Huy, D.H.; Seelen, E.; Liem-Nguyen, V. Removal mechanisms of cadmium and lead ions in contaminated water by stainless steel slag obtained from scrap metal recycling. J. Water Process. Eng. 2020, 36, 101369. [Google Scholar] [CrossRef]
- Shi, L.; Wang, N.; Hu, X.; Yin, D.; Wu, C.; Liang, H.; Cao, W.; Cao, H. Acute toxic effects of lead (Pb2+) exposure to rare minnow (Gobiocypris rarus) revealed by histopathological examination and transcriptome analysis. Environ. Toxicol. Pharmacol. 2020, 78, 103385. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, F.; Sun, Y.; Xu, X.; Chen, X.; Pan, B.; Sun, D.; Qian, J. Bacterial cellulose derived paper-like purifier with multifunctionality for water decontamination. Chem. Eng. J. 2019, 371, 730–737. [Google Scholar] [CrossRef]
- Beni, A.A.; Esmaeili, A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A review. Environ. Technol. Innov. 2020, 17, 100503. [Google Scholar] [CrossRef]
- Bazzazzadeh, R.; Soudi, M.; Valinassab, T.; Moradlou, O. Kinetics and equilibrium studies on biosorption of hexavalent chromium from leather tanning wastewater by Sargassum tenerrimum from Chabahar-Bay Iran. Algal Res. 2020, 48, 101896. [Google Scholar] [CrossRef]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; Sastre de Vicente, M.E. Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.; Young, B.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sust. Energ. Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- de Souza Coração, A.C.; dos Santos, F.S.; Duarte, J.A.D.; Lopes-Filho, E.A.P.; de Paula, J.C.; Rocha, L.M.; Krepsky, N.; Fiaux, S.B.; Teixeira, V.L. What do we know about the utilization of the Sargassum species as biosorbents of trace metals in Brazil? J. Environ. Chem. Eng. 2020, 8, 103941. [Google Scholar] [CrossRef]
- Júnior, W.N.; Silva, M.; Vieira, M. Competitive fixed-bed biosorption of Ag (I) and Cu (II) ions on Sargassum filipendula seaweed waste. J. Water Process. Eng. 2020, 36, 101294. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Teo, T.T.; Balasubramanian, R.; Joshi, U.M. Application of Sargassum biomass to remove heavy metal ions from synthetic multi-metal solutions and urban storm water runoff. J. Hazard. Mater. 2009, 164, 1019–1023. [Google Scholar] [CrossRef]
- Johnson, D.R.; Ko, D.S.; Franks, J.S.; Moreno, P.; Sanchez-Rubio, G. The Sargassum Invasion of the Eastern Caribbean and Dynamics of the Equatorial North Atlantic. In Proceedings of the 65th Gulf and Caribbean Fisheries Institute, Santa Marta, Colombia, 5–9 November 2013; pp. 102–103. [Google Scholar]
- Silva, R.; Mendoza, E.; Mariño-Tapia, I.; Martínez, M.L.; Escalante, E. An artificial reef improves coastal protection and provides a base for coral recovery. J. Coast. Res. 2016, 75, 467–471. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic Sargassum belt. Sci. 2019, 365, 83–87. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.J.W. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar]
- Hannachi, Y.; Hafidh, A. Biosorption potential of Sargassum muticum algal biomass for methylene blue and lead removal from aqueous medium. Int. J. Environ. Sci. Technol. 2020, 17, 3875–3890. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Li, Y.; Li, X.; You, L.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydr. Polym. 2020, 230, 115647. [Google Scholar] [CrossRef]
- Krzek, J.; Apola, A.; Stolarczyk, M.; Rzeszutko, W. Spectrophotometric determination of Pb(II), Fe(III) and Bi(III) in complexes with 1,2-diaminocyclohexane-N,N,N’,N’-tetraacetic acid (DACT). Acta Pol. Pharm. 2007, 64, 3–8. [Google Scholar]
- Qin, C.; Du, Y.; Xiao, L. Effect of hydrogen peroxide treatment on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2002, 76, 211–218. [Google Scholar] [CrossRef]
- Momeni, S.; Nabipour, I. A simple green synthesis of palladium nanoparticles with Sargassum alga and their electrocatalytic activities towards hydrogen peroxide. Appl. Biochem. Biotech. 2015, 176, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Kannan, S. FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 341–351. [Google Scholar]
- Singh, P.K.; Singh, R.L. Bio-removal of azo dyes: A review. Int. J. Appl. Sci. Biotechnol. 2017, 5, 108–126. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, C.; Wang, C.; Sun, J.; Wang, X.; Li, X. A TiO2 modified abiotic–biotic process for the degradation of the azo dye methyl orange. Rsc Adv. 2015, 5, 58704–58712. [Google Scholar] [CrossRef]
- Tabaraki, R.; Sadeghinejad, N. Biosorption of six basic and acidic dyes on brown alga Sargassum ilicifolium: Optimization, kinetic and isotherm studies. Water Sci. Technol. 2017, 75, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- Arulmozhi, K.; Mythili, N. Studies on the chemical synthesis and characterization of lead oxide nanoparticles with different organic capping agents. Aip Adv. 2013, 3, 122122. [Google Scholar] [CrossRef]
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K.; Marycz, K. Using ICP-OES and SEM-EDX in biosorption studies. Microchim. Acta 2011, 172, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, A.; Saremnia, B.; Kalantari, M. Removal of mercury (II) from aqueous solutions by biosorption on the biomass of Sargassum glaucescens and Gracilaria corticata. Arab. J. Chem. 2015, 8, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Seolatto, A.A.; Martins, T.D.; Bergamasco, R.; Tavares, C.R.G.; Cossich, E.S.; da Silva, E. Biosorption study of Ni2+ and Cr3+ by Sargassum filipendula: Kinetics and equilibrium. Braz. J. Chem. Eng. 2014, 31, 211–227. [Google Scholar] [CrossRef]
- Mahmood, Z.; Zahra, S.; Iqbal, M.; Raza, M.A.; Nasir, S. Comparative study of natural and modified biomass of Sargassum sp. for removal of Cd 2+ and Zn 2+ from wastewater. Appl. Water Sci. 2017, 7, 3469–3481. [Google Scholar] [CrossRef] [Green Version]
- Veit, M.T.; da Cunha Gonçalves, G.; Fagundes-Klen, M.R.; Da Silva, E.A.; Tavares, C.R.G.; Honorio, J.F. Organic leaching and metal removal with < i> Sargassum filipendula. Acta Sci. Technol. 2014, 36, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, S.; Tvn, P.; Krishnan, S. Removal Of Nickel And Copper From Aqueous Solution By Sargassum SP.To Remediates Metal Contaminated Industrial Effluents. Int. J. Appl. Bioeng. 2009, 3, 26–30. [Google Scholar] [CrossRef]

















| R2 | K (s−1) | Removal (%) | ||
|---|---|---|---|---|
| Methylene Blue | ||||
| Chemical Modification | ||||
| Dry | 0.9740 | 0.129 | 80.86 | |
| Treated | 0.9959 | 0.131 | 95.99 | |
| Sargassum Mass | ||||
| 1 g | 0.9905 | 0.060 | 93.67 | |
| 3 g | 0.9959 | 0.082 | 92.25 | |
| 7 g | 0.9955 | 0.163 | 95.39 | |
| Mechanical Treatment | ||||
| crushing | 0.982 | 0.160 | 92.79 | |
| grinding | 0.984 | 0.185 | 87.42 | |
| Filter Diameter | ||||
| 0.5 in | 0.982 | 0.177 | 94.64 | |
| 1 in | 0.993 | 0.134 | 96.01 | |
| 1.5 in | 0.970 | 0.157 | 92.74 | |
| Number of Layers | ||||
| 2 | 0.998 | 0.124 | 97.91 | |
| 3 | 0.988 | 0.139 | 98.02 | |
| 4 | 0.993 | 0.161 | 98.25 | |
| Flow Rate | ||||
| 9 mL/s | 0.998 | 0.112 | 99.64 | |
| 5 mL/s | 0.989 | 0.139 | 98.45 | |
| 3 mL/s | 0.999 | 0.208 | 99.74 | |
| Humidity | ||||
| 20% | 0.999 | 0.398 | 99.34 | |
| 30% | 0.999 | 0.347 | 99.79 | |
| 50% | 0.999 | 0.440 | 99.91 | |
| Methyl Orange | ||||
| ** | 0.982 | 0.150 | 25.66 | |
| Methyl Red | ||||
| ** | 0.999 | 0.222 | 67.97 |
| Sargassum Species | Substances Removed | Pollutant Inlet Concentration (PPM) | % Removal | Device | Reference |
|---|---|---|---|---|---|
| Sargassum natanes and Sargassum fluitanes | Methylene blue | 4 | 99 | Filter | This work |
| Methyl orange | 3 | 25 | |||
| Methyl red | 6 | 68 | |||
| Pb2+ | 50 | 95 | |||
| Sargassum muticum | Methylene blue | 50 | 96 * | Bed column | [30] |
| Pb2+ | 50 | 96 * | |||
| Sargassum glaucescens | Hg2+ | 0.2–1 | 95 | Rotary shaker | [44] |
| Sargassum filipendula | Ni2+ | 59 and 147 | 30 and 45 | Agitation | [45] |
| Cr3+ | 52 and 139 | 88 and 52 | |||
| Sargassum sp. | Cd2+ | 5 | 95 | Agitation | [46] |
| Zn2+ | 5 | 90 | |||
| Sargassum filipendula | Cr3+ | 121 | 79 | Agitation | [47] |
| Ni2+ | 205 | 52 | |||
| Sargassum sp. | Cu2+ | 346 | 87 | Agitation | [48] |
| Ni2+ | 182 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.R.; Hernández-Carteño, J.; Estévez, M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp. J. Mar. Sci. Eng. 2020, 8, 899. https://doi.org/10.3390/jmse8110899
López-Miranda JL, Silva R, Molina GA, Esparza R, Hernandez-Martinez AR, Hernández-Carteño J, Estévez M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp. Journal of Marine Science and Engineering. 2020; 8(11):899. https://doi.org/10.3390/jmse8110899
Chicago/Turabian StyleLópez-Miranda, J. Luis, Rodolfo Silva, Gustavo A. Molina, Rodrigo Esparza, A. R. Hernandez-Martinez, J. Hernández-Carteño, and Miriam Estévez. 2020. "Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum Spp." Journal of Marine Science and Engineering 8, no. 11: 899. https://doi.org/10.3390/jmse8110899