Metal Ions and Hydroperoxide Content: Main Drivers of Coastal Lipid Autoxidation in Riverine Suspended Particulate Matter and Higher Plant Debris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tracers Used
2.2. Experimental
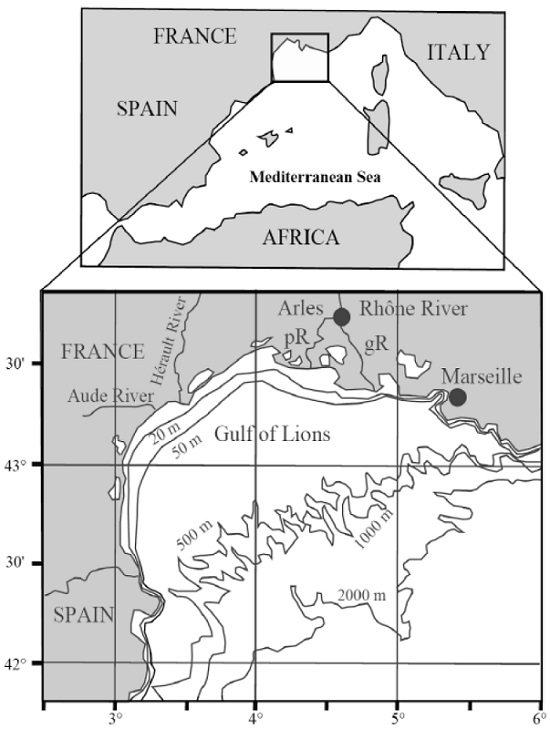
2.2.1. Rhône SPM Samples
- Seawater
- Ultra-pure water
- Ultra-pure water with added Cu2+ (2.4 μM of CuSO4)
- Ultra-pure water with added Fe2+ (1.0 mM of Mohr’s salt)
2.2.2. Terrestrial Vascular Plant Samples
2.2.3. Chemical Treatment of the Samples
3. Results
3.1. Rhone SPM Incubations
3.2. Plant Debris Incubations
4. Discussion
4.1. Rhone River SPM Incubations
4.2. Leaf Debris Incubations
4.3. Hydroperoxide Content
4.4. Environmental Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schaich, K.M. Lipid oxidation: Theoretical aspects. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Wiley: New York, NY, USA, 2005; pp. 269–355. [Google Scholar]
- Rontani, J.-F.; Rabourdin, A.; Marchand, D.; Aubert, C. Photochemical oxidation and autoxidation of chlorophyll phytyl side chain in senescent phytoplanktonic cells: Potential sources of several acyclic isoprenoid compounds in the marine environment. Lipids 2003, 38, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Simic, M.G.; Karel, M. Autoxidation in Food and Biological Systems; Springer: New York, NY, USA, 1980. [Google Scholar]
- Stocks, J.; Dormandy, T.L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. 1971, 20, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Galeron, M.-A.; Amiraux, R.; Charrière, B.; Radakovitch, O.; Raimbault, P.; Garcia, N.; Lagadec, V.; Vaultier, F.; Rontani, J.-F. Seasonal survey of the composition and degradation state of particulate organic matter in the Rhône River using lipid tracers. Biogeosciences 2015, 12, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Rontani, J.-F.; Charrière, B.; Sempéré, R.; Doxaran, D.; Vaultier, F.; Vonk, J.E.; Volkman, J.K. Degradation of sterols and terrigenous organic matter in waters of the Mackenzie Shelf, Canadian Arctic. Org. Geochem. 2014, 75, 61–73. [Google Scholar] [CrossRef]
- Bawn, C.E.H. Free radical reactions in solution initiated by heavy metal ions. Discuss. Faraday Soc. 1953, 14, 181–190. [Google Scholar] [CrossRef]
- Miller, D.M.; Buettner, G.R.; Aust, S.D. Transition metals as catalysts of ‘autoxidation’ reactions. Free Radic. Biol. Med. 1990, 8, 95–108. [Google Scholar] [CrossRef]
- Galeron, M.-A.; Vaultier, F.; Rontani, J.-F. Oxidation products of α- and β-amyrins: Potential tracers of abiotic degradation of vascular plant organic matter in aquatic environments. Environ. Chem. 2016, 13, 732–744. [Google Scholar] [CrossRef]
- Galeron, M.-A.; Volkman, J.K.; Rontani, J.-F. Oxidation products of betulin: New tracers of abiotic degradation of higher plant material in the environment. Org. Geochem. 2016, 91, 31–42. [Google Scholar] [CrossRef]
- Ollivier, P.; Radakovitch, O.; Hamelin, B. Major and trace element partition and fluxes in the Rhône River. Chem. Geol. 2011, 285, 15–31. [Google Scholar] [CrossRef]
- Marchand, D.; Rontani, J.-F. Characterization of photo-oxidation and autoxidation products of phytoplanktonic monounsaturated fatty acids in marine particulate matter and recent sediments. Org. Geochem. 2001, 32, 287–304. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Charrière, B.; Forest, A.; Heussner, S.; Vaultier, F.; Petit, M.; Delsaut, N.; Fortier, L.; Sempéré, R. Intense photooxidative degradation of planktonic and bacterial lipids in sinking particles collected with sediment traps across the Canadian Beaufort Shelf (Arctic Ocean). Biogeosciences 2012, 9, 4787–4802. [Google Scholar] [CrossRef]
- Mihara, S.; Tateba, H. Photosensitized oxygenation reactions of phytol and its derivatives. J. Org. Chem. 1986, 51, 1142–1144. [Google Scholar] [CrossRef]
- Shulkin, V.; Bogdanova, N. Mobilization of metals from riverine suspended matter in seawater. Mar. Chem. 2003, 83, 157–167. [Google Scholar] [CrossRef]
- Milward, G.E.; Liu, Y.P. Modelling metal desorptions kinetics in estuaries. Sci. Total Environ. 2003, 314–316, 613–623. [Google Scholar] [CrossRef]
- Eriksson, E.; Welander, P. On a mathematical model of the carbon cycle in nature. Tellus 1956, 8, 155–175. [Google Scholar] [CrossRef]
- Baes, C.F.J.; Goeller, H.E.; Olson, J.S.; Rotty, R.M. Global Carbon Dioxide Problem. 1976 (An Oak Ridge National Lab. (TN) Report (USA)). Available online: http://www.osti.gov/scitech/biblio/7145150 (accessed on 20 May 2016).
- Baes, C.F.; Goeller, H.E.; Olson, J.S.; Rotty, R.M. Carbon dioxide and climate: The uncontrolled experiment: Possibly severe consequences of growing CO2 release from fossil fuels require a much better understanding of the carbon cycle, climate change, and the resulting impacts on the atmosphere. Am. Sci. 1977, 65, 310–320. [Google Scholar]
- Sundquist, E.T. The global carbon dioxide budget. Science 1993, 259, 934–941. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007. Available online: https://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_synthesis_report.htm (accessed on 19 May 2016).
- Sarmiento, J.L.; Gruber, N. Sinks for Anthropogenic Carbon. Phys. Today 2002, 55, 30–36. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeron, M.-A.; Radakovitch, O.; Charrière, B.; Rontani, J.-F. Metal Ions and Hydroperoxide Content: Main Drivers of Coastal Lipid Autoxidation in Riverine Suspended Particulate Matter and Higher Plant Debris. J. Mar. Sci. Eng. 2016, 4, 50. https://doi.org/10.3390/jmse4030050
Galeron M-A, Radakovitch O, Charrière B, Rontani J-F. Metal Ions and Hydroperoxide Content: Main Drivers of Coastal Lipid Autoxidation in Riverine Suspended Particulate Matter and Higher Plant Debris. Journal of Marine Science and Engineering. 2016; 4(3):50. https://doi.org/10.3390/jmse4030050
Chicago/Turabian StyleGaleron, Marie-Aimée, Olivier Radakovitch, Bruno Charrière, and Jean-François Rontani. 2016. "Metal Ions and Hydroperoxide Content: Main Drivers of Coastal Lipid Autoxidation in Riverine Suspended Particulate Matter and Higher Plant Debris" Journal of Marine Science and Engineering 4, no. 3: 50. https://doi.org/10.3390/jmse4030050