Characterization of Intertidal Macrofaunal Communities of Two Sandy Beaches under Different Anthropogenic Pressures
Abstract
:1. Introduction
2. Materials and Methods
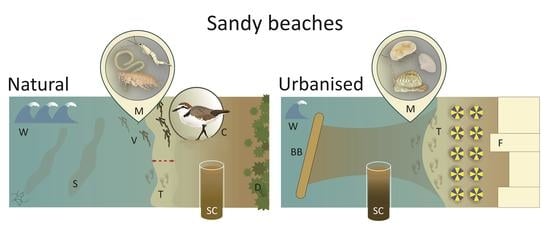
2.1. Study Areas
2.2. Sampling Design and Fieldwork
2.3. Data Elaboration
3. Results
3.1. Temporal Variations in Abundance of Living and Non-Living Components
3.2. Data elaboration
3.3. Environmental Parameters
4. Discussion
4.1. Overview of the Characteristics of the Two Sites
4.2. Living and Non-Living Components in the Collected Sediments
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aller, R.C. Benthic fauna and biogeochemical processes in marine sediments: The role of burrow structures. In Nitrogen Cycling in Coastal Marine Environments; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1988; pp. 301–338. [Google Scholar]
- Meysman, F.J.; Galaktionov, O.S.; Middelburg, J.J. Irrigation patterns in permeable sediments induced by burrow ventilation: A case study of Arenicola marina. Mar. Ecol. Prog. Ser. 2005, 303, 195–212. [Google Scholar] [CrossRef]
- Huhta, V. The role of soil fauna in ecosystems: A historical review. Pedobiologia 2007, 50, 489–495. [Google Scholar] [CrossRef]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, A.F.; DeWitt, T.H. Geochemical ecosystem engineering by the mud shrimp Upogebia pugettensis (Crustacea: Thalassinidae) in Yaquina Bay, Oregon: Density-dependent effects on organic matter remineralization and nutrient cycling. Limnol. Oceanogr. 2009, 54, 1911–1932. [Google Scholar] [CrossRef]
- Huettel, M.; Rusch, A. Transport and degradation of phytoplankton in permeable sediment. Limnol. Oceanogr. 2000, 45, 534–549. [Google Scholar] [CrossRef] [Green Version]
- Lloret, J.; Marín, A. The contribution of benthic macrofauna to the nutrient filter in coastal lagoons. Mar. Pollut. Bull. 2011, 62, 2732–2740. [Google Scholar] [CrossRef]
- Rhoads, D.C. The influence of deposit-feeding benthos on water turbidity and nutrient recycling. Am. J. Sci. 1973, 273, 1–22. [Google Scholar] [CrossRef]
- Aller, R.C. The effects of animal-sediment interactions on geochemical processes near the sediment-water interface. In Estuarine Interactions; Academic Press: San Diego, CA, USA, 1978; pp. 157–172. [Google Scholar]
- Graf, G. Benthic-pelagic coupling: A benthic view. Oceanogr. Mar. Biol. 1992, 30, 149–190. [Google Scholar]
- Koike, I.; Mukai, H. Oxygen and inorganic nitrogen contents and fluxes in burrows of the shrimps Callianassa japonica and Upogebia major. Mar. Ecol. Prog. Ser. 1983, 12, 185–190. [Google Scholar] [CrossRef]
- Widdicombe, S.; Austen, M.C.; Kendall, M.A.; Warwick, R.M.; Jones, M.B. Bioturbation as a mechanism for setting and maintaining levels of diversity in subtidal macrobenthic communities. Hydrobiologia 2000, 440, 369–377. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Meysman, F.J.; Middelburg, J.J.; Heip, C.H. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol. Evol. 2006, 21, 688–695. [Google Scholar] [CrossRef]
- Passarelli, C.; Olivier, F.; Paterson, D.M.; Meziane, T.; Hubas, C. Organisms as cooperative ecosystem engineers in intertidal flats. J. Sea Res. 2014, 92, 92–101. [Google Scholar] [CrossRef]
- De Smet, B.; D’Hondt, A.S.; Verhelst, P.; Fournier, J.; Godet, L.; Desroy, N.; Rabaut, M.; Vincx, M.; Vanaverbeke, J. Biogenic reefs affect multiple components of intertidal soft-bottom benthic assemblages: The Lanice conchilega case study. Estuar. Coast. Shelf Sci. 2015, 152, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Carlton, J.T.; Hodder, J. Maritime mammals: Terrestrial mammals as consumers in marine intertidal communities. Mar. Ecol. Prog. Ser. 2003, 256, 271–286. [Google Scholar] [CrossRef] [Green Version]
- Aarif, K.M.; Nefla, A.; Nasser, M.; Prasadan, P.K.; Athira, T.R.; Muzaffar, S.B. Multiple environmental factors and prey depletion determine declines in abundance and timing of departure in migratory shorebirds in the west coast of India. Glob. Ecol. Conserv. 2021, 26, e01518. [Google Scholar] [CrossRef]
- Castro, M.; Masero, J.A.; Megina, C.; Amat, J.A.; Pérez-Hurtado, A. Energy and macronutrient assimilation efficiencies of Snowy Plover (Charadrius alexandrinus) feeding on the polychaete ragworm (Nereis diversicolor). Auk 2008, 125, 368–373. [Google Scholar] [CrossRef]
- Masero, J.A.; Pérez-Huratdo, A.; Castro, M.; Arroyo, G.M. Complementary use of intertidal mudflats and adjacent salinas by foraging waders. Ardea 2000, 88, 177–191. [Google Scholar]
- Wright, L.; Short, A. Morphodynamic variability of surf zones and beaches: A synthesis. Mar. Geol. 1984, 56, 93–118. [Google Scholar] [CrossRef]
- Cerrano, C.; Arillo, A.; Bavestrello, G.; Benatti, U.; Calcinai, B.; Cattaneo-Vietti, R.; Cortesogno, L.; Gaggero, L.; Giovine, M.; Puce, S.; et al. Organism–quartz interactions in structuring benthic communities: Towards a marine bio-mineralogy? Ecol. Lett. 1999, 2, 1–3. [Google Scholar] [CrossRef]
- Dexter, D.M. Sandy Beach Community Structure: The Role of Exposure and Latitude. J. Biogeogr. 1992, 19, 59. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Barboza, F.R.; Defeo, O. Global diversity patterns in sandy beach macrofauna: A biogeographic analysis. Sci. Rep. 2015, 5, 14515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, T.H.; Rosenberg, R. Feast and famine: Structuring factors in marine benthic communities. Symp. Br. Ecol. Soc. 1987, 7, 373–395. [Google Scholar]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. 1978, 16, 229–311. [Google Scholar]
- Josefson, A.B.; Widbom, B. Differential response of benthic macrofauna and meiofauna to hypoxia in the Gullmar Fjord basin. Mar. Biol. 1988, 100, 31–40. [Google Scholar] [CrossRef]
- Schückel, U.; Ehrich, S.; Kröncke, I. Temporal variability of three different macrofauna communities in the northern North Sea. Estuar. Coast. Shelf Sci. 2010, 89, 1–11. [Google Scholar] [CrossRef]
- Jayaraj, K.A.; Josia, J.; Kumar, P.D. Infaunal macrobenthic community of soft bottom sediment in a tropical shelf. J. Coast. Res. 2008, 24, 708–718. [Google Scholar] [CrossRef]
- Ólafsson, E.B.; Peterson, C.H.; Ambrose, W.G., Jr. Does recruitment limitation structure populations and communities of macro-invertebrates in marine soft sediments: The relative significance of pre-and post-settlement processes. Oceanogr. Mar. Biol. 1994, 32, 65–109. [Google Scholar]
- Rossi, F.; Forster, R.; Montserrat, F.; Ponti, M.; Terlizzi, A.; Ysebaert, T.; Middelburg, J.J. Human trampling as short-term disturbance on intertidal mudflats: Effects on macrofauna biodiversity and population dynamics of bivalves. Mar. Boil. 2007, 151, 2077–2090. [Google Scholar] [CrossRef] [Green Version]
- Schlacher, T.A.; Carracher, L.K.; Porch, N.; Connolly, R.M.; Olds, A.D.; Gilby, B.L.; Ekanayake, K.B.; Maslo, B.; Weston, M.A. The Early Shorebird Will Catch Fewer Invertebrates on Trampled Sandy Beaches. PLoS ONE 2016, 11, e0161905. [Google Scholar] [CrossRef]
- Zielinski, S.; Botero, C.M.; Yanes, A. To clean or not to clean? A critical review of beach cleaning methods and impacts. Mar. Pollut. Bull. 2019, 139, 390–401. [Google Scholar] [CrossRef]
- Thrush, S.F.; Hewitt, J.E.; Kraan, C.; Lohrer, A.M.; Pilditch, C.A.; Douglas, E. Changes in the location of biodiversity—ecosystem function hot spots across the seafloor landscape with increasing sediment nutrient loading. Proc. R. Soc. B Boil. Sci. 2017, 284, 20162861. [Google Scholar] [CrossRef] [Green Version]
- Defeo, O.; Elliott, M. The ‘triple whammy’ of coasts under threat—Why we should be worried. Mar. Pollut. Bull. 2021, 163, 111832. [Google Scholar] [CrossRef]
- Bessa, F.; Cunha, D.; Gonçalves, S.; Marques, J.C. Sandy beach macrofaunal assemblages as indicators of anthropogenic impacts on coastal dunes. Ecol. Indic. 2013, 30, 196–204. [Google Scholar] [CrossRef]
- Bessa, F.; Gonçalves, S.; Franco, J.N.; André, J.N.; Cunha, P.P.; Marques, J.C. Temporal changes in macrofauna as response indicator to potential human pressures on sandy beaches. Ecol. Indic. 2014, 41, 49–57. [Google Scholar] [CrossRef]
- Reis, R.D.S.; Rizzo, A.E. Human Trampling Effect on Benthic Fauna of Sandy Beaches with Different Intensities of Use in Rio De Janeiro, Brazil. Oecologia Aust. 2019, 23, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Vieira, J.V.; Borzone, C.A.; Lorenzi, L.; Carvalho, F.G.D. Human impact on the benthic macrofauna of two beach environments with different morphodynamic characteristics in southern Brazil. Braz. J. Oceanogr. 2012, 60, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Afghan, A.; Cerrano, C.; Luzi, G.; Calcinai, B.; Puce, S.; Mantas, T.P.; Roveta, C.; di Camillo, C.G. Main anthropogenic impacts on benthic macrofauna of sandy beaches: A review. J. Mar. Sci. Eng. 2020, 8, 405. [Google Scholar] [CrossRef]
- Defeo, O.; de Alava, A. Effects of human activities on long-term trends in sandy beach populations: The wedge clam Donax hanleyanus in Uruguay. Mar. Ecol. Prog. Ser. 1995, 123, 73–82. [Google Scholar] [CrossRef]
- Lucrezi, S.; Schlacher, T.A. Impacts of off-road vehicles (ORVs) on burrow architecture of ghost crabs (Genus Ocypode) on sandy beaches. J. Environ. Manag. 2010, 45, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Martínez, M.J.; Ruíz-Delgado, M.C.; Sanchez-Moyano, J.E.; Garcia-García, F.J. Response of intertidalsandy-beach macrofauna to human trampling: An urban vs. natural beach system approach. Mar. Environ. Res. 2015, 103, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Veloso, V.G.; Silva, E.S.; Caetano, C.H.S.; Cardoso, R.S. Comparison between the macroinfauna of urbanizedand protected beaches in Rio de Janeiro State, Brazil. Biol. Conserv. 2006, 127, 510–515. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Iaciofano, D.; Lo Turco, V.; Potortì, A.G.; Rando, R.; Arizza, V.; Di Stefano, V. First Assessment of Plasticizers in Marine Coastal Litter-Feeder Fauna in the Mediterranean Sea. Toxics 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Veloso, V.G.; Sallorenzo, I.A.; Ferreira, B.C.A.; De Souza, G.N. Atlantorchestoidea brasiliensis (Crustacea: Amphipoda) as an indicator of disturbance caused by urbanization of a beach ecosystem. Braz. J. Oceanogr. 2010, 58, 13–21. [Google Scholar] [CrossRef]
- Machado, P.M.; Suciu, M.C.; Costa, L.L.; Tavares, D.C.; Zalmon, I.R. Tourism impacts on benthic communities of sandy beaches. Mar. Ecol. 2017, 38, e12440. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Lucrezi, S.; Connolly, R.M.; Peterson, C.H.; Gilby, B.L.; Maslo, B.; Olds, A.D.; Walker, S.J.; Leon, J.X.; Huijbers, C.M.; et al. Human threats to sandy beaches: A meta-analysis of ghost crabs illustrates global anthropogenic impacts. Estuar. Coast. Shelf Sci. 2016, 169, 56–73. [Google Scholar] [CrossRef]
- Parlagreco, L.; Melito, L.; Devoti, S.; Perugini, E.; Soldini, L.; Zitti, G.; Brocchini, M. Monitoring for coastal resilience: Preliminary data from five italian sandy beaches. Sensors 2019, 19, 1854. [Google Scholar] [CrossRef] [Green Version]
- Briggs, M.J.; Thompson, E.F.; Vincent, C.L. Wave diffraction around breakwater. J. Waterw. Port Coast. Ocean Eng. 1995, 121, 23–35. [Google Scholar] [CrossRef]
- ARPAM. Relazione Triennale sulla Qualità dei Corpi Idrici Marino Costieri della Regione Marche—Triennio 2018–2020. Agenzia Regionale per la Protezione Ambientale—Regione Marche (ARPAM). 2021. Available online: https://www.arpa.marche.it/images/PUBBLICAZIONI/Corpi_idrici_marino_costieri_2018-2020.pdf (accessed on 2 September 2022).
- ARTA. MONITORAGGIO delle Acque Marino Costiere della Regione Abruzzo Classificazione Triennio 2018–2020 (D. Lgs. 152/06). Agenzia Regionale per la Tutela dell’Ambiente—Regione Abruzzo (ARTA). 2021. Available online: https://www.artaabruzzo.it/download/pubblicazioni/20220908_relazione_triennio_2018_-_2020_rev_0.pdf (accessed on 12 September 2022).
- Available online: https://www.portaleacque.salute.gov.it/PortaleAcquePubblico/mappa.do (accessed on 12 September 2022).
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. Geol. J. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Available online: www.mareografico.it (accessed on 10 January 2020).
- Available online: www.meteopesca.com (accessed on 10 January 2020).
- Available online: www.ilmeteo.it/portale/meteo-mare (accessed on 10 January 2020).
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial. PRIMER-E Plymouth. PRIMER-E Ltd: Ivybridge, UK, 2015. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Clarke, K.R.; Green, R.H. Statistical Design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- CLARKE, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Di Camillo, C.G.; Coppari, M.; Bartolucci, I.; Bo, M.; Betti, F.; Bertolino, M.; Calcinai, B.; Cerrano, C.; De Grandes, G.; Bavestrello, G. Temporal variations in growth and reproduction of Tedania anhelans and Chondrosia reniformis in the North Adriatic Sea. Hydrobiologia 2012, 687, 299–313. [Google Scholar] [CrossRef]
- Corte, G.N.; Checon, H.H.; Esmaeili, Y.S.; Defeo, O.; Turra, A. Evaluation of the effects of urbanization and environmental features on sandy beach macrobenthos highlights the importance of submerged zones. Mar. Pollut. Bull. 2022, 182, 113962. [Google Scholar] [CrossRef]
- Bertasi, F.; Colangelo, M.A.; Abbiati, M.; Ceccherelli, V.U. Effects of an artificial protection structure on the sandy shore macrofaunal community: The special case of Lido di Dante (Northern Adriatic Sea). Hydrobiologia 2007, 586, 277–290. [Google Scholar] [CrossRef]
- Karapurkar, D.; Hegde, V.S.; Ramakrishnan, R. Sediment dispersal pattern along an engineered micro-tidal tropical estuarine beach. J. Earth Syst. Sci. 2022, 131, 13. [Google Scholar] [CrossRef]
- Munari, C.; Corbau, C.; Simeoni, U.; Mistri, M. Coastal defence through low crested breakwater structures: Jumping out of the frying pan into the fire? Mar. Pollut. Bull. 2011, 62, 1641–1651. [Google Scholar] [CrossRef]
- Vona, I.; Gray, M.W.; Nardin, W. The Impact of Submerged Breakwaters on Sediment Distribution along Marsh Boundaries. Water 2020, 12, 1016. [Google Scholar] [CrossRef] [Green Version]
- ARPAM. Relazione Annuale sulla Qualità delle Acque di Balneazione 2019. Agenzia Regionale per la Protezione Ambientale—Regione Marche (ARPAM). 2019. Available online: https://www.arpa.marche.it/images/PUBBLICAZIONI/ICONE/RelBalneazione2019.pdf (accessed on 12 September 2022).
- Targusi, M.; la Porta, B.; Lattanzi, L.; la Valle, P.; Loia, M.; Paganelli, D.; Pazzini, A.; Proietti, R.; Nicoletti, L. Beach nourishment using sediments from relict sand deposit: Effects on subtidal macrobenthic communities in the Central Adriatic Sea (Eastern Mediterranean Sea-Italy). Mar. Environ. Res. 2019, 144, 186–193. [Google Scholar] [CrossRef]
- Southward, A.J.; Southward, E.C.; Dando, P.R.; Hughes, J.A.; Kennicutt, M.C.; Herrera-Alcala, J.; Leahy, Y. Behaviour and feeding of the nassariid gastropod Cyclope neritea, abundant at hydrothermal brine seeps off Milos (Aegean Sea). J. Mar. Biol. Assoc. 1997, 77, 753. [Google Scholar] [CrossRef]
- Ugolini, A.; Ungherese, G.; Somigli, S.; Galanti, G.; Baroni, D.; Borghini, F.; Cipriani, N.; Nebbiai, M.; Passaponti, M.; Focardi, S. The amphipod Talitrus saltator as a bioindicator of human trampling on sandy beaches. Mar. Environ. Res. 2008, 65, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, G.; Acquavita, A.; Celio, M.; Ciriaco, S.; Cirilli, S.; Fernetti, M.; Pugliese, N. Ostracod Fauna: Eyewitness to Fifty Years of Anthropic Impact in the Gulf of Trieste. A Potential Key to the Future Evolution of Urban Ecosystems. Sustainability 2020, 12, 6954. [Google Scholar] [CrossRef]
- Casu, D.; Ceccherelli, G.; Castelli, A. Immediate effects of experimental human trampling on mid-upper intertidal benthic invertebrates at the Asinara Island MPA (NW Mediterranean). Hydrobiologia 2006, 555, 271–279. [Google Scholar] [CrossRef]
- Tewfik, A.; Bell, S.S.; McCann, K.S.; Morrow, K. Predator diet and trophic position modified with altered habitat morphology. PLoS ONE 2016, 11, e0147759. [Google Scholar] [CrossRef]
- Imperio, S.; Nardelli, R.; Serra, L. Protocollo per il Monitoraggio del Fratino—ISPRA (Istituto Superiore per la Protezione e la Ricerca Ambientale). 2020. Available online: https://www.torredelcerrano.it/wp-content/uploads/2021/01/Protocollo-per-il-monitoraggio-del-fratino-sui-litorali.pdf (accessed on 12 September 2022).
- Rodil, I.F.; Lastra, M. Beyond physical control: Macrofauna community diversity across sandy beaches and its relationship with secondary production. Estuar. Coast. Shelf Sci. 2022, 277, 108083. [Google Scholar] [CrossRef]
- Colombini, I.; Chelazzi, L. Influence of marine allochthonous input on sandy beach communities. Oceanogr. Mar. Biol. Annu. Rev. 2003, 41, 123–127. [Google Scholar]
- Directive 2000/60/EC Of The European Parliament And Of The Council Establishing A Framework For The Community Action In The Field Of Water Policy (EU Water Framework Directive, WFD). Available online: https://ec.europa.eu/environment/water/water-framework/index_en.html (accessed on 12 September 2022).
- Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32008L0056&qid=1664874023839 (accessed on 12 September 2022).
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31992L0043 (accessed on 12 September 2022).
- Serrano Giné, D.; Jurado Rota, J.; Pérez Albert, M.Y.; Bonfill Cerveró, C. The Beach Crowding Index: A tool for assessing social carrying capacity of vulnerable beaches. Prof. Geogr. 2018, 70, 412–422. [Google Scholar] [CrossRef]
- Costa, L.L.; Fanini, L.; Zalmon, I.R.; Defeo, O.; McLachlan, A. Cumulative stressors impact macrofauna differentially according to sandy beach type: A meta-analysis. J. Environ. Manag. 2022, 307, 114594. [Google Scholar] [CrossRef]
- Costa, L.L.; Zalmon, I.R.; Fanini, L.; Defeo, O. Macroinvertebrates as indicators of human disturbances on sandy beaches: A global review. Ecol. Indic. 2020, 118, 106764. [Google Scholar] [CrossRef]
- Laurino, I.R.; Checon, H.H.; Corte, G.N.; Turra, A. Does coastal armoring affect biodiversity and its functional composition on sandy beaches? Mar. Environ. Res. 2022, 181, 105760. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Jiang, S.C. Alterations of the human skin microbiome after ocean water exposure. Mar. Pollut. Bull. 2019, 145, 595–603. [Google Scholar] [CrossRef]
- Rodil, I.F.; Harris, L.R.; Lucrezi, S.; Cerrano, C. Sandy Beach Management and Conservation: The Integration of Economic, Social and Ecological Values. In Sandy Beaches as Endangered Ecosystems; CRC Press: London, UK, 2022; pp. 251–294. [Google Scholar]






| Phylum | Class | Superorder | Order | Family | Taxon | PAL | TC | Analysed as |
|---|---|---|---|---|---|---|---|---|
| Annelida | Polychaeta | - | - | Capitellidae | Capitella capitata (Fabricius, 1780) | X | Polychaeta | |
| Annelida | Polychaeta | - | - | Magelonidae | Magelona sp. | X | Polychaeta | |
| Annelida | Polychaeta | - | Phyllodocida | Glyceridae | Glycera sp. | X | X | Polychaeta |
| Annelida | Polychaeta | - | Phyllodocida | Phyllodocidae | Eteone sp. | X | Polychaeta | |
| Annelida | Polychaeta | - | Phyllodocida | Sigalionidae | Sigalion mathildae Audouin & Milne Edwards, 1832 | X | X | Polychaeta |
| Annelida | Polychaeta | - | Spionida | Spionidae | Scolelepis sp. | X | X | Polychaeta |
| Annelida | Polychaeta | - | Spionida | Spionidae | Spionidae | X | X | Polychaeta |
| Annelida | Polychaeta | - | Terebellida | Cirratulidae | Cirratulidae | X | Polychaeta | |
| Annelida | Polychaeta | - | Terebellida | Terebellidae | Terebellidae | X | Polychaeta | |
| Arthropoda | Malacostraca | Eucarida | Decapoda | Callianassidae | Gilvossius candidus (Olivi, 1792) | X | Decapoda | |
| Arthropoda | Malacostraca | Eucarida | Decapoda | Diogenidae | Diogenes pugilator (Roux, 1829) | X | Decapoda | |
| Arthropoda | Malacostraca | Peracarida | Amphipoda | Bathyporeiidae | Bathyporeia guilliamsoniana (Spence Bate, 1857) | X | X | Amphipoda |
| Arthropoda | Malacostraca | Peracarida | Amphipoda | Caprellidae | Caprellidae | X | Amphipoda | |
| Arthropoda | Malacostraca | Peracarida | Amphipoda | Gammaridae | Echinogammarus stocki G. Karaman, 1970 | X | X | Amphipoda |
| Arthropoda | Malacostraca | Peracarida | Amphipoda | Oedicerotidae | Pontocrates altamarinus (Spence Bate & Westwood, 1862) | X | X | Amphipoda |
| Arthropoda | Malacostraca | Peracarida | Cumacea | Bodotriidae | Cumopsis sp. | X | X | Cumacea |
| Arthropoda | Malacostraca | Peracarida | Cumacea | Pseudocumatidae | Pseudocuma sp. | X | X | Cumacea |
| Arthropoda | Malacostraca | Peracarida | Isopoda | Cirolanidae | Eurydice spinigera Hansen, 1890 | X | X | Isopoda |
| Arthropoda | Malacostraca | Peracarida | Isopoda | Idoteidae | Idotea pelagica Leach, 1816 | X | X | Isopoda |
| Arthropoda | Malacostraca | Peracarida | Mysida | Mysidae | Paramysis (Longidentia) helleri (G.O. Sars, 1877) | X | X | Mysida |
| Arthropoda | Malacostraca | Peracarida | Tanaidacea | Apseudidae | Apseudopsis latreillii (Milne Edwards, 1828) | X | X | Tanaidacea |
| Arthropoda | Ostracoda | - | - | - | Ostracoda | X | X | Ostracoda |
| Echinodermata | Ophiuroidea | - | - | - | Ophiuroidea | X | Ophiuroidea | |
| Echinodermata | Ophiuroidea | Ophintegrida | Ophiurida | Amphiuridae | Amphiura chiajei Forbes, 1843 | X | Ophiuroidea | |
| Mollusca | Bivalvia | - | - | - | Bivalvia ≤ 5 mm | X | X | Bivalvia ≤ 5 mm |
| Mollusca | Bivalvia | Imparidentia | Cardiida | Donacidae | Donax trunculus Linnaeus, 1758 | X | X | Bivalvia > 5 mm |
| Mollusca | Bivalvia | Imparidentia | Myida | Corbulidae | Lentidium mediterraneum (O. G. Costa, 1830) | X | X | Lentidium |
| Mollusca | Bivalvia | Imparidentia | Venerida | Veneridae | Chamelea gallina (Linnaeus, 1758) | X | X | Bivalvia > 5 mm |
| Mollusca | Gastropoda | - | Littorinimorpha | Naticidae | Neverita josephinia Risso, 1826 | X | Gastropoda | |
| Mollusca | Gastropoda | - | Neogastropoda | Nassariidae | Tritia neritea (Linnaeus, 1758) | X | Gastropoda |
| Items | Description |
|---|---|
| Polychaetes soft tubes | Empty tubes made of cemented sand grains belonging to polychaetes |
| Hard tubes | Carbonatic tubes of serpulids and tusk shells (Scaphopoda) |
| Tube fragments | Other fragments of tubes of unidentified organisms |
| Ophiuroid vertebrae | Ophiuroid skeletal elements (complete vertebrae) |
| Sea urchin fragments | Fragments of tests > 5 mm |
| Sea urchin spines | Only frgments including the basal part by which the spine is attached to the test. |
| Gastropod shells | Entire, empty shells |
| Anthropogenic litter | Every kind of rubbish |
| Vegetal detritus | Vegetal detritus both of marine and land origin (Wood, seeds, plant and algal fragments > 5 mm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Camillo, C.G.; Luzi, G.; Danial, A.; Di Florio, L.; Calcinai, B.; Lo Brutto, S.; de Oliveira, J.L.S.M.; Fumanti, A.; Cerrano, C. Characterization of Intertidal Macrofaunal Communities of Two Sandy Beaches under Different Anthropogenic Pressures. J. Mar. Sci. Eng. 2022, 10, 1976. https://doi.org/10.3390/jmse10121976
Di Camillo CG, Luzi G, Danial A, Di Florio L, Calcinai B, Lo Brutto S, de Oliveira JLSM, Fumanti A, Cerrano C. Characterization of Intertidal Macrofaunal Communities of Two Sandy Beaches under Different Anthropogenic Pressures. Journal of Marine Science and Engineering. 2022; 10(12):1976. https://doi.org/10.3390/jmse10121976
Chicago/Turabian StyleDi Camillo, Cristina Gioia, Giorgia Luzi, Afghan Danial, Luciano Di Florio, Barbara Calcinai, Sabrina Lo Brutto, Jéssica Luana Santana Mendonça de Oliveira, Agnese Fumanti, and Carlo Cerrano. 2022. "Characterization of Intertidal Macrofaunal Communities of Two Sandy Beaches under Different Anthropogenic Pressures" Journal of Marine Science and Engineering 10, no. 12: 1976. https://doi.org/10.3390/jmse10121976