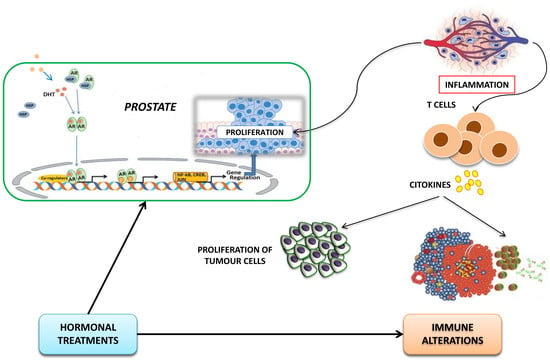
Immune Modulation in Prostate Cancer Patients Treated with Androgen Receptor (AR)-Targeted Therapy
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Diagnostic Criteria of Autoimmune Disease
2.3. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
3.2. Emergence of Autoimmune Disorders Before Starting AR-Directed Therapies
3.3. Autoimmune Comorbidities and Clinical Outcome to Abiraterone and Enzalutamide
3.4. Risk of Developing Second Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, A.; Conteduca, V.; Zoubeidi, A.; Beltran, H. Biological Evolution of Castration-resistant Prostate Cancer. Eur. Urol. Focus 2019, 5, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B., Jr.; Saad, F.; et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, V.; Caffo, O.; Galli, L.; Maugeri, A.; Scarpi, E.; Maines, F.; Chiuri, V.E.; Lolli, C.; Kinspergher, S.; Schepisi, G.; et al. Association among metabolic syndrome, inflammation, and survival in prostate cancer. Urol. Oncol. 2018, 36, e1–e240. [Google Scholar] [CrossRef]
- Conteduca, V.; Caffo, O.; Derosa, L.; Veccia, A.; Petracci, E.; Chiuri, V.E.; Santoni, M.; Santini, D.; Fratino, L.; Maines, F.; et al. Metabolic syndrome in castration-resistant prostate cancer patients treated with abiraterone. Prostate 2015, 75, 1329–1338. [Google Scholar] [CrossRef]
- Conteduca, V.; Di Lorenzo, G.; Tartarone, A.; Aieta, M. The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: An unresolved controversy. Crit. Rev. Oncol. Hematol. 2013, 86, 42–51. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019, 165, 196–206. [Google Scholar] [CrossRef]
- Medina, G.; Vera-Lastra, O.; Peralta-Amaro, A.L.; Jiménez-Arellano, M.P.; Saavedr, M.A.; Cruz-Domínguez, M.P.; Jara, L.J. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol. Res. 2018, 133, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Kalina, J.L.; Neilson, D.S.; Comber, A.P.; Rauw, J.M.; Alexander, A.S.; Vergidis, J.; Lum, J.J. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, M.D.; McNeel, D.G. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum. Immunol. 2010, 71, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Salman, H.; Bergman, M.; Blumberger, N.; Djaldetti, M.; Bessler, H. Do androgen deprivation drugs affect the immune cross-talk between mononuclear and prostate cancer cells? Biomed. Pharmacother. 2014, 68, 21–24. [Google Scholar] [CrossRef]
- Fijak, M.; Schneider, E.; Klug, J.; Bhushan, S.; Hackstein, H.; Schuler, G.; Wygrecka, M.; Gromoll, J.; Meinhardt, A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: Evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol. 2011, 186, 5162–5172. [Google Scholar] [CrossRef] [Green Version]
- Radojevic, K.; Arsenovic-Ranin, N.; Kosec, D.; Pesic, V.; Pilipovic, I.; Perisic, M.; Plecas-Solarovic, B.; Leposavic, G. Neonatal castration affects intrathymic kinetics of T- cell differentiation and the spleen T-cell level. J. Endocrinol. 2007, 192, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Moore, M.L.; Grayson, J.M.; Dubey, P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012, 72, 1975–1985. [Google Scholar] [CrossRef] [Green Version]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Ponzetta, A.; Inforzato, A.; Jaillon, S. Innate immunity, inflammation and tumour progression: Double-edged swords. J. Intern. Med. 2019, 285, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Kissick, H.T.; Sanda, M.G.; Dunn, L.K.; Pellegrini, K.L.; On, S.T.; Noel, J.K.; Arredouani, M.S. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 9887–9992. [Google Scholar] [CrossRef] [Green Version]
- Drake, C.G.; Doody, A.D.; Mihalyo, M.A.; Huang, C.T.; Kelleher, E.; Ravi, S.; Hipkiss, E.L.; Flies, D.B.; Kennedy, E.P.; Long, M.; et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005, 7, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.M.; Yu, C.P.; Chuang, H.C.; Wu, C.T.; Hsu, R.J. Androgen deprivation therapy for prostate cancer and the risk of autoimmune diseases. Prostate Cancer Prostatic Dis. 2019, 22, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Krasnova, A.; Nead, K.T.; Choueiri, T.K.; Hu, J.C.; Hoffman, K.E.; Yu, J.B.; Spratt, D.E.; Feng, F.Y.; Trinh, Q.D.; et al. Androgen deprivation therapy and risk of rheumatoid arthritis in patients with localized prostate cancer. Ann. Oncol. 2018, 29, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Farolfi, A.; Conteduca, V.; Martignano, F.; De Lisi, D.; Ravaglia, G.; Rossi, L.; Menna, C.; Bellia, S.R.; Barone, D.; et al. Immunotherapy for Prostate Cancer: Where We Are Headed. Int. J. Mol. Sci. 2017, 18, 2627. [Google Scholar] [CrossRef] [Green Version]
- Pu, Y.; Xu, M.; Liang, Y.; Yang, K.; Guo, Y.; Yang, X.; Fu, Y.X. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumour relapse. Sci. Transl. Med. 2016, 8, 333ra347. [Google Scholar] [CrossRef] [Green Version]
- Gamat, M.; McNeel, D.G. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr. Relat. Cancer 2017, 24, T297–T310. [Google Scholar] [CrossRef]
- Solanki, A.A.; Bossi, A.; Efstathiou, J.A.; Lock, D.; Mondini, M.; Ramapriyan, R.; Welsh, J.; Kang, J. Combining Immunotherapy with Radiotherapy for the Treatment of Genitourinary Malignancies. Eur. Urol. Oncol. 2019, 2, 79–87. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Lolli, C.; Caffo, O.; Scarpi, E.; Aieta, M.; Conteduca, V.; Maines, F.; Bianchi, E.; Massari, F.; Veccia, A.; Chiuri, V.E.; et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front. Pharmacol. 2016, 7, 376. [Google Scholar] [CrossRef] [Green Version]
- Conteduca, V.; Crabb, S.J.; Jones, R.J.; Caffo, O.; Elliott, T.; Scarpi, E.; Fabbri, P.; Derosa, L.; Massari, F.; Numico, G.; et al. Persistent Neutrophil to Lymphocyte Ratio >3 during Treatment with Enzalutamide and Clinical Outcome in Patients with Castration-Resistant Prostate Cancer. PLoS ONE 2016, 11, e0158952. [Google Scholar] [CrossRef]
- Gillessen, S.; Templeton, A.; Marra, G.; Kuo, Y.F.; Valtorta, E.; Shahinian, V.B. Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J. Natl. Cancer Inst. 2010, 102, 1760–1770. [Google Scholar] [CrossRef] [Green Version]
- Krasnow, R.E.; Rodríguez, D.; Nagle, R.T.; Mossanen, M.; Kibel, A.S.; Chang, S.L. The impact of age at the time of radiotherapy for localized prostate cancer on the development of second primary malignancies. Urol. Oncol. 2018, 36, e11–e500. [Google Scholar] [CrossRef]
- Moschini, M.; Zaffuto, E.; Karakiewicz, P.I.; Andrea, D.D.; Foerster, B.; Abufaraj, M.; Soria, F.; Mattei, A.; Montorsi, F.; Briganti, A.; et al. External Beam Radiotherapy Increases the Risk of Bladder Cancer When Compared with Radical Prostatectomy in Patients Affected by Prostate Cancer: A Population-based Analysis. Eur. Urol. 2019, 75, 319–328. [Google Scholar] [CrossRef]



| Characteristics | n = 844 |
|---|---|
| Age, years | |
| median value (IQR) | 70 (63–75) |
| Gleason score, n (%) | |
| 6–7 | 297 (40.1) |
| ≥8 | 444 (59.9) |
| Missing/Unknown | 103 |
| Visceral metastases, n (%) | |
| No | 752 (89.1) |
| Yes | 92 (10.9) |
| ECOG PS, n (%) | |
| 0–1 | 788 (93.4) |
| 2 | 56 (6.6) |
| Baseline PSA, ng/mL | |
| median value (IQR) | 35.42 (8.64–115.40) |
| HSPC duration, months median value (IQR) ADT for HSPC, n (%) | 29 (14–59) 844 (100) |
| CRPC duration, months median value (IQR) Prior therapeutic lines for CRPC, n (%) | 22 (13–39) |
| 1–2 | 764 (90.5) |
| >2 | 80 (9.5) |
| Potent AR-directed therapies for CRPC, n (%) | 844 (100) |
| Abiraterone | 477 (56.5) |
| Chemotherapy-naive | 189 (39.6) |
| Post-docetaxel | 288 (60.4) |
| Enzalutamide | 367 (43.5) |
| Chemotherapy-naive | 170 (46.3) |
| Post-docetaxel | 197 (53.7) |
| Comorbidities | Total n (%) |
|---|---|
| No | 277 (32.8) |
| Yes | 567 (67.2) |
| Arterial hypertension | 351 (41.6) |
| Metabolic syndrome | 105 (12.4) |
| Dyslipidemia | 99 (11.7) |
| Diabetes mellitus type 2 | 95 (11.3) |
| Ischemic cardiopathy | 82 (9.7) |
| Arrhythmia | 73 (8.6) |
| Vascular disease | 38 (4.5) |
| Gastrointestinal disease | 35 (4.1) |
| Neurological disease | 35 (4.1) |
| Chronic obstructive pulmonary disease | 28 (3.3) |
| Non-autoimmune thyropathy | 19 (2.2) |
| Nephropathy | 17 (2.0) |
| Autoimmune disease | 36 (4.3) |
| Arthritis (8 rheumatoid, 5 psoriatic) | 13 (1.5) |
| Autoimmune thyroiditis | 12 (1.4) |
| Chronic inflammatory bowel disease (3 ulcerative colitis, 1 Crohn’s disease) | 4 (0.5) |
| Psoriasis | 4 (0.5) |
| Systemic vasculitis (2 giant cell arteritis, 1 vasculitis, 1 unspecified) | 3 (0.4) |
| Second tumor (38 solid, 9 hematological) | 47 (5.6) |
| PFS | OS | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Autoimmune disease (yes versus no) | 1.37 (0.92–2.05) | 0.119 | 1.63 (1.03–2.57) | 0.037 |
| Age (continuous variable) | 1.002 (0.991–1.014) | 0.690 | 1.015 (1.001–1.029) | 0.042 |
| Visceral metastasis (yes versus no) | 1.27 (0.97–1.67) | 0.085 | 1.82 (1.35–2.45) | <0.0001 |
| ECOG PS (2 versus 0–1) | 2.09 (1.53–2.86) | <0.0001 | 3.30 (2.37–4.58) | <0.0001 |
| Pretreatment log PSA (continuous variable) | 1.29 (1.23–1.36) | <0.0001 | 1.41 (1.32–1.49) | <0.0001 |
| Previous chemotherapy (yes versus no) | 1.87 (1.55–2.25) | <0.0001 | 1.74 (1.38–2.19) | <0.0001 |
| Other comorbidities * (yes versus no) | 1.04 (0.87–1.25) | 0.645 | 1.05 (0.84–1.32) | 0.638 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conteduca, V.; Caffo, O.; Scarpi, E.; Sepe, P.; Galli, L.; Fratino, L.; Maines, F.; Chiuri, V.E.; Santoni, M.; Zanardi, E.; et al. Immune Modulation in Prostate Cancer Patients Treated with Androgen Receptor (AR)-Targeted Therapy. J. Clin. Med. 2020, 9, 1950. https://doi.org/10.3390/jcm9061950
Conteduca V, Caffo O, Scarpi E, Sepe P, Galli L, Fratino L, Maines F, Chiuri VE, Santoni M, Zanardi E, et al. Immune Modulation in Prostate Cancer Patients Treated with Androgen Receptor (AR)-Targeted Therapy. Journal of Clinical Medicine. 2020; 9(6):1950. https://doi.org/10.3390/jcm9061950
Chicago/Turabian StyleConteduca, Vincenza, Orazio Caffo, Emanuela Scarpi, Pierangela Sepe, Luca Galli, Lucia Fratino, Francesca Maines, Vincenzo Emanuele Chiuri, Matteo Santoni, Elisa Zanardi, and et al. 2020. "Immune Modulation in Prostate Cancer Patients Treated with Androgen Receptor (AR)-Targeted Therapy" Journal of Clinical Medicine 9, no. 6: 1950. https://doi.org/10.3390/jcm9061950