Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products
Abstract
:1. Introduction
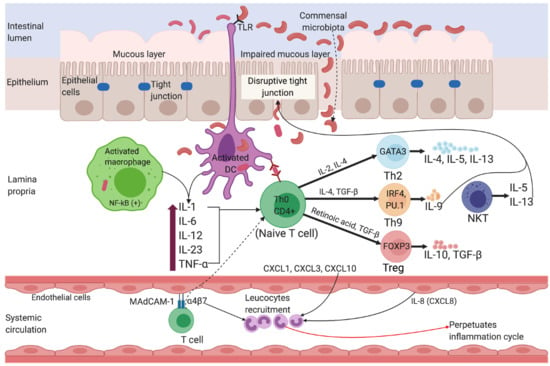
2. Diagnosis and Pathophysiology of Inflammatory Bowel Disease
2.1. Ulcerative Colitis
2.2. Crohn’s Disease
3. Causes and Risk Factors of Inflammatory Bowel Disease
3.1. Genetics
3.2. Environmental Factors
3.3. Microbiota
3.4. Diet and Smoking
3.5. Sleep Deprivation, Stress, and Physical Inactivity
3.6. Appendectomy
3.7. Antibiotic Use
4. Current Treatment Options for IBD
4.1. Conventional Treatments
4.1.1. Small Molecule Drugs
4.1.2. Biologics
4.1.3. Surgical Treatment
4.2. Alternative Treatment Options for IBD
4.2.1. Botanicals Used for Treating IBD
4.2.2. Helminth Therapies
4.3. Challenges in the Treatment Regimens and Management of IBD
4.3.1. Diagnosis
4.3.2. Accessibility and Affordability of IBD Treatments
5. Anti-Inflammatory Activities of Natural Products
5.1. Techniques and Biological Assays Used in the Discovery of Anti-Inflammatory Extracts and Drug Leads
5.2. Anti-Inflammatory Activities of Plant Extracts and Compounds
5.3. Fungal-Derived Anti-Inflammatory Drug Leads
5.4. Helminth-Derived Anti-Inflammatories
5.5. Anti-Inflammatory Peptides
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mulder, D.J.; Noble, A.J.; Justinich, C.J.; Duffin, J.M. A tale of two diseases: The history of inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Aufses, A.H. The history of Crohn’s Disease. Surg. Clin. N. Am. 2001, 81, 1–11. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol 2019, 5, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Aniwan, S.; Tremaine, W.J.; Raffals, L.E.; Kane, S.V.; Loftus, E.V., Jr. Antibiotic Use and New-Onset Inflammatory Bowel Disease in Olmsted County, Minnesota: A Population-Based Case-Control Study. J. Crohn’s Colitis 2018, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Hui, A.J.; Wong, T.C.; Leung, V.K.; Tsang, S.W.; Yu, H.H.; et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013, 145, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Inflammatory Bowel Disease National Action Plan 2019. Available online: https://www.crohnsandcolitis.com.au/site/wp-content/uploads/National-Action-Plan-FINAL-08-03-2019.pdf (accessed on 12 December 2019).
- Yang, Y.; Owyang, C.; Wu, G.D. East Meets West: The Increasing Incidence of Inflammatory Bowel Disease in Asia as a Paradigm for Environmental Effects on the Pathogenesis of Immune-Mediated Disease. Gastroenterology 2016, 151, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Asakura, K.; Nishiwaki, Y.; Inoue, N.; Hibi, T.; Watanabe, M.; Takebayashi, T. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J. Gastroenterol. 2009, 44, 659–665. [Google Scholar] [CrossRef]
- Kwak, M.S.; Cha, J.M.; Lee, H.H.; Choi, Y.S.; Seo, S.I.; Ko, K.J.; Park, D.I.; Kim, S.H.; Kim, T.J. Emerging trends of inflammatory bowel disease in South Korea: A nationwide population-based study. J. Gastroenterol. Hepatol. 2019, 34, 1018–1026. [Google Scholar] [CrossRef]
- Yen, H.H.; Weng, M.T.; Tung, C.C.; Wang, Y.T.; Chang, Y.T.; Chang, C.H.; Shieh, M.J.; Wong, J.M.; Wei, S.C. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: A nationwide populationbased study. Intest. Res. 2019, 17, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, A.; Sedgwick, D.M.; Drummond, J. Morbidity of juvenile onset inflammatory bowel disease: Effects on education and employment in early adult life. Gut 1994, 35, 665–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappelman, M.D.; Rifas-Shiman, S.L.; Porter, C.Q.; Ollendorf, D.A.; Sandler, R.S.; Galanko, J.A.; Finkelstein, J.A. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology 2008, 135, 1907–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longobardi, T.; Jacobs, P.; Bernstein, C.N. Work losses related to inflammatory bowel disease in the United States results from the National Health Interview Survey. Am. J. Gastroenterol. 2003, 98, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- M’Koma, A.E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 2013, 6, 33–47. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Improving Inflammatory Bowel Disease Care across Australia. Available online: https://www.crohnsandcolitis.com.au/site/wp-content/uploads/PwC-report-2013.pdf (accessed on 10 January 2020).
- Jeong, D.Y.; Kim, S.; Son, M.J.; Son, C.Y.; Kim, J.Y.; Kronbichler, A.; Lee, K.H.; Shin, J.I. Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review. Autoimmun. Rev. 2019, 18, 439–454. [Google Scholar] [CrossRef]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Ruel, J.; Ruane, D.; Mehandru, S.; Gower-Rousseau, C.; Colombel, J.F. IBD across the age spectrum: Is it the same disease? Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 88–98. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Guindi, M.; Riddell, R.H. Indeterminate colitis. J. Clin. Pathol. 2004, 57, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Tremaine, W.J. Diagnosis and Treatment of Intermediate Colitis. Gastroenterol. Hepatol. 2011, 7, 826–828. [Google Scholar]
- Teixeira, F.V.; Hosne, R.S.; Sobrado, C.W. Management of ulcerative colitis: A clinical update. J. Coloproctol. 2015, 35, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.crohnsandcolitis.org.uk/about-crohns-and-colitis/publications/ulcerative-colitis (accessed on 25 November 2019).
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Eliakim, R.; Magro, F.; Maaser, C.; Chowers, Y.; Geboes, K.; Mantzaris, G.; Reinisch, W.; Colombel, J.F.; Vermeire, S.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: Definitions and diagnosis. J. Crohn’s Colitis 2012, 6, 965–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Roediger, W.E.; Moore, J.; Babidge, W. Colonic Sulfite in Pathogenesis and Treatment of Ulcerative Colitis. Dig. Dis. Sci. 1997, 42, 1571–1579. [Google Scholar] [CrossRef]
- Bamias, G.; Nyce, M.R.; Sarah, A.; Cominelli, F. New Concepts in the Pathophysiology of Inflammatory Bowel Disease. Ann. Intern. Med. 2005, 143, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Neurath, M.; Boirivant, M.; Klein, J.S.; De La Motte, C.; Strong, S.A.; Fiocchi, C.; Strober, W. Disparate CD4+ Lamina Propria (LP)Lymphokine Secretion Profiles in Inflammatory Bowel Disease Crohn’s Disease LP Cells Manifest Increased Secretion of IFN-7, Whereas UlcerativeColitisLPCellsManifestIncreasedSecretion ofIL-5. J. Immunol. 1996, 157, 1261–1270. [Google Scholar] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, F.; Peter, F.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis That Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Fromm, A.; Gitter, A.H.; Mankertz, J.; Schulzke, J.D. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: Effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal. Immunol. 2008, 1, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Nalleweg, N.; Chiriac, M.T.; Podstawa, E.; Lehmann, C.; Rau, T.T.; Atreya, R.; Krauss, E.; Hundorfean, G.; Fichtner-Feigl, S.; Hartmann, A.; et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015, 64, 743–755. [Google Scholar] [CrossRef] [Green Version]
- Hokari, R.; Kato, S.; Matsuzaki, K.; Iwai, A.; Kawaguchi, A.; Nagao, S.; Miyahara, T.; Itoh, K.; Sekizuka, E.; Nagata, H.; et al. Involvement of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of granulomatous colitis in rats. Clin. Exp. Immunol. 2001, 126, 259–262. [Google Scholar] [CrossRef]
- Arihiro, S.; Ohtani, H.; Suzuki, M.; Murata, M.; Ejima, C.; Oki, M.; Kinouchi, Y.; Fukushima, K.; Sasaki, I.; Nakamura, S.; et al. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn’s disease. Pathol. Int. 2002, 52, 367–374. [Google Scholar] [CrossRef]
- Jussila, A.; Virta, L.J.; Pukkala, E.; Farkkila, M.A. Mortality and causes of death in patients with inflammatory bowel disease: A nationwide register study in Finland. J. Crohn’s Colitis 2014, 8, 1088–1096. [Google Scholar] [CrossRef] [Green Version]
- Winther, K.V.; Jess, T.; Langholz, E.; Munkholm, P.; Binder, V. Survival and Cause-Specific Mortality in Ulcerative Colitis: Follow-up of a Population-Based Cohort in Copenhagen County. Gastroenterology 2003, 125, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, T.; Jarvis, K.; Patel, J. Diagnosis and Management of Crohn’s Disease. Am. Fam. Physician 2011, 84, 1365–1375. [Google Scholar] [PubMed]
- Van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Røseth, A.G.; Aadland, E.; Jahnsen, J.; Raknerud, N. Assessment of disease activity in ulcerative colitis by faecal calprotein, a novel granulocyte marker protein. Digestion 1997, 58, 176–180. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Li, F.; Ma, J.; Geng, S.; Wang, J.; Liu, J.; Zhang, J.; Sheng, X. Fecal calprotectin concentrations in healthy children aged 1-18 months. PLoS ONE 2015, 10, e0119574. [Google Scholar] [CrossRef] [Green Version]
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef]
- Kolls, J.K.; Linden, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Inoue, N.; Sato, T.; Okamoto, S.; Hisamatsu, T.; Kishi, Y.; Sakuraba, A.; Hitotsumatsu, O.; Ogata, H.; Koganei, K.; et al. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut 2004, 53, 1303–1308. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, J.F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Herrick, E.M.; Voller, A. Suppression of autoimmune disease in NZB and (NZB × NZW) F1 hybrid mice by infection with malaria. Nature 1970, 226, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.M.; Herrick, E.M.; Voller, A. Can parasitic infection suppress autoimmune disease? Proc. R. Soc. Med. 1970, 63, 19–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewell, D.L.; Reinke, E.K.; Hogan, L.H.; Sandor, M.; Fabry, Z. Immunoregulation of CNS autoimmunity by helminth and mycobacterial infections. Immunol. Lett. 2002, 82, 101–110. [Google Scholar] [CrossRef]
- Rook, G.A.; Brunet, L.R. Microbes, immunoregulation, and the gut. Gut 2005, 54, 317–320. [Google Scholar] [CrossRef]
- Feeney, M.A.; Murphy, F.; Clegg, A.J.; Trebble, T.M.; Sharer, N.M.; Snook, J.A. A case–control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2002, 14, 529–534. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Tysk, C.; Lindberg, E.; Järnerot, G.; Floderus-Myrhed, B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut 1988, 29, 990–996. [Google Scholar] [CrossRef] [Green Version]
- Thompson, N.P.; Driscoll, R.; Pounder, R.E.; Wakefield, A.J. Genetics versus environment in inflammatory bowel disease: Results of a British twin study. BMJ 1996, 312, 95–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfvarson, J.; Bodin, L.; Tysk, C.; Lindberg, E.; Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: A long-term follow-up of concordance and clinical characteristics. Gastroenterology 2003, 124, 1767–1773. [Google Scholar] [CrossRef]
- Halme, L.; Paavola-Sakki, P.; Turunen, U.; Lappalainen, M.; Färkkilä, M.; Kontula, K. Family and twin studies in inflammatory bowel disease. World J. Gastroenterol. 2006, 12, 3668–3672. [Google Scholar] [CrossRef]
- Khalili, H.; Huang, E.S.; Ananthakrishnan, A.N.; Higuchi, L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012, 61, 1686–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Beissert, S.; Schwarz, T.; Schwarz, A. Phenotypic and functional characterization of ultraviolet radiation-induced regulatory T cells. J. Immunol. 2008, 180, 3065–3071. [Google Scholar] [CrossRef] [Green Version]
- Maeda, S.S.; Saraiva, G.L.; Hayashi, L.F.; Cendoroglo, M.S.; Ramos, L.R.; Correa Mde, P.; Henrique de Mesquita, C.; Lazaretti-Castro, M. Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in Sao Paulo, Brazil: The Sao PAulo Vitamin D Evaluation Study (SPADES). Dermatoendocrinol 2013, 5, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Froicu, M.; Weaver, V.; Wynn, T.A.; McDowell, M.A.; Welsh, J.E.; Cantorna, M.T. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol. Endocrinol. 2003, 17, 2386–2392. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Pinsk, V.; Lemberg, D.A.; Grewal, K.; Barker, C.C.; Schreiber, R.A.; Jacobson, K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am. J. Gastroenterol. 2007, 102, 1077–1083. [Google Scholar] [CrossRef]
- Probert, C.S.; Jayanthi, V.; Pinder, D.; Wicks, A.C.; Mayberry, J.F. Epidemiological study of ulcerative proctocolitis in Indian migrants and the indigenous population of Leicestershire. Gut 1992, 33, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchimol, E.I.; Mack, D.R.; Guttmann, A.; Nguyen, G.C.; To, T.; Mojaverian, N.; Quach, P.; Manuel, D.G. Inflammatory bowel disease in immigrants to Canada and their children: A population-based cohort study. Am. J. Gastroenterol. 2015, 110, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Svanes, C.; Janson, C.; Sigsgaard, T.; Johannessen, A.; Gislason, T.; Jogi, R.; Omenaas, E.; Forsberg, B.; Toren, K.; et al. Place of upbringing in early childhood as related to inflammatory bowel diseases in adulthood: A population-based cohort study in Northern Europe. Eur. J. Epidemiol. 2014, 29, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoetendal, E.G.; Puylaert, P.G.; Ou, J.; Vipperla, K.; Brouard, F.M.; Ruder, E.H.; Newton, K.; Carbonero, F.; Gaskins, H.R.; de Vos, W.M.; et al. Sa1968 Distinct Microbiotas Are Present in Urban and Rural Native South Africans, and in African Americans. Gastroenterology 2013, 144. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, T.S.; Kedia, S.; Rampal, R.; Saxena, S.; Bag, S.; Mitra, R.; Dayal, M.; Mehta, O.; Surendranath, A.; et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High Altitude Areas. Sci. Rep. 2018, 8, 10104. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Nagalingam, N.A.; Lynch, S.V. Role of the microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2012, 18, 968–984. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, 79. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Dore, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermudez-Humaran, L.G.; Langella, P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jick, H.; Walker, A.M. Cigarettesmokingandulcerativecolitis. N. Engl. J. Med. 1983, 308, 261–263. [Google Scholar] [CrossRef]
- Gyde, S.; Prior, P.; Dew, M.J.; Saunders, V.; Waterhouse, J.A.; Allan, R.N. Mortality in ulcerative colitis. Gastroenterology 1982, 83, 36–43. [Google Scholar] [CrossRef]
- Vessey, M.; Jewell, D.; Smith, A.; Yeates, D.; McPherson, K. Chronic inflammatory bowel disease, cigarette smoking, and use of oral contraceptives: Findings in a large cohort study of women of childbearing age. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 1101–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullan, R.D.; Rhodes, J.; Ganesh, S.; Mani, V.; Morris, J.S.; Williams, G.T.; Newcombe, R.G.; Russel, M.A.H.; Feyerabend, C.; Thomas, G.A.O.; et al. Transdermal Nicotine for Active Ulcerative Colitis. N. Engl. J. Med. 1994, 330, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Fujiwara, Y.; Iwakura, N.; Shiba, M.; Watanabe, K.; Kamata, N.; Yamagami, H.; Tanigawa, T.; Watanabe, T.; Tominaga, K.; et al. Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. Springerplus 2016, 5, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Richter, J.M.; Schernhammer, E.S.; Chan, A.T. Sleep duration affects risk for ulcerative colitis: A prospective cohort study. Clin. Gastroenterol. Hepatol. 2014, 12, 1879–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikocka-Walus, A.; Pittet, V.; Rossel, J.B.; von Kanel, R. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 829–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikocka-Walus, A.; Massuger, W.; Knowles, S.R.; Moore, G.T.; Buckton, S.; Connell, W.; Pavli, P.; Raven, L.; Andrews, J.M. Psychological distress is highly prevalent in inflammatory bowel disease: A survey of psychological needs and attitudes. JGH Open 2019. [Google Scholar] [CrossRef] [Green Version]
- Lerebours, E.; Gower-Rousseau, C.; Merle, V.; Brazier, F.; Debeugny, S.; Marti, R.; Salomez, J.L.; Hellot, M.F.; Dupas, J.L.; Colombel, J.F.; et al. Stressful life events as a risk factor for inflammatory bowel disease onset: A population-based case-control study. Am. J. Gastroenterol. 2007, 102, 122–131. [Google Scholar] [CrossRef]
- Swanson, G.R.; Burgess, H.J.; Keshavarzian, A. Sleep disturbances and inflammatory bowel disease: A potential trigger for disease flare? Expert Rev. Clin. Immunol. 2011, 7, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Mardini, H.E.; Kip, K.E.; Wilson, J.W. Crohn’s Disease: A Two-Year Prospective Study of the Association Between Psychological Distress and Disease Activity. Dig. Dis. Sci. 2004, 49, 492–497. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.L.; Andreoli, A.; Luzi, C.; Arca, M.; Berto, E.; Milite, G.; Marcheggiano, A. Stress and exacerbation in Ulcerative Colitis: A prospective study of patients enrolled in remission. Am. J. Gastroenterol. 2000, 95, 1213–1220. [Google Scholar] [CrossRef]
- Radford-Smith, G.L. What is the importance of appendectomy in the natural history of IBD? Inflamm. Bowel Dis. 2008, 14 (Suppl. 2), 72–74. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Mizoguchi, E.; Chiba, C.; Bhan, A.K. Role of Appendix in the Development of Inflammatory Bowel Disease in TCR-c~ Mutant Mice. J. Exp. Med. 1996, 184, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombaerts, P.M.; Mizoguchi, E.; Grusby, M.J.; Glimcher, L.H.; Bhan, A.K.; Tonegawa, S. Spontaneous Development of Inflammatory Bowel Disease in T Cell Receptor Mutant Mice. Cell 1993, 76, 276–282. [Google Scholar] [CrossRef]
- Radford-Smith, G.L.; Edwards, J.E.; Purdie, D.M.; Pandeya, N.; Watson, M.; Martin, N.G.; Green, A.; Newman, B.; Florin, T.H. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn’s disease. Gut 2002, 51, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.H.; Cheung, D.Y.; Kim, T.H.; Jun, E.J.; Lee, I.K.; Kim, J.I.; Cho, S.H.; Park, S.H.; Han, J.Y.; Kim, J.K. Remission of ulcerative colitis after appendectomy: A case report. Korean J. Gastroenterol. 2010, 56, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Svanstrom, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am. J. Gastroenterol. 2011, 106, 2133–2142. [Google Scholar] [CrossRef]
- Virta, L.; Auvinen, A.; Helenius, H.; Huovinen, P.; Kolho, K.L. Association of repeated exposure to antibiotics with the development of pediatric Crohn’s disease—A nationwide, register-based finnish case-control study. Am. J. Epidemiol. 2012, 175, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Garcia Rodriguez, L.A.; Ruigomez, A.; Panes, J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006, 130, 1588–1594. [Google Scholar] [CrossRef]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 2012, 130, 794–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.S.; Luben, R.; Bergmann, M.M.; Boeing, H.; Olsen, A.; Tjonneland, A.; Overvad, K.; Kaaks, R.; Kennedy, H.; Khaw, K.T.; et al. Aspirin in the aetiology of Crohn’s disease and ulcerative colitis: A European prospective cohort study. Aliment. Pharmacol. Ther. 2011, 34, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Knutson, D.; Greenberg, G.; Cronau, H. Management of Crohn’s Disease—A Practical Approach. Am. Fam. Physician 2003, 68, 707–714. [Google Scholar]
- Faubion, W.A.J.; Loftus, E.V.J.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001, 121, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Truelove, S.C.; Witts, L.J. Cortisone in Ulcerative Colitis. Br. Med. J. 1955, 2, 1041–1048. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L. Filgotinib in Crohn’s Disease: JAK Is Back. Gastroenterology 2017, 153, 603–605. [Google Scholar] [CrossRef]
- Olivera, P.; Danese, S.; Peyrin-Biroulet, L. Next generation of small molecules in inflammatory bowel disease. Gut 2017, 66, 199–209. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Fernandez-Clotet, A.; Castro-Poceiro, J.; Panes, J. Tofacitinib for the treatment of ulcerative colitis. Expert Rev. Clin. Immunol. 2018, 14, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Hanauer, S.B. Controversies With Aminosalicylates in Inflammatory Bowel Disease. Rev. Gastroenterol. Disord. 2004, 4, 104–117. [Google Scholar] [PubMed]
- Rudrapatna, V.A.; Velayos, F. Biosimilars for the Treatment of Inflammatory Bowel Disease. Pract. Gastroenterol. 2019, 43, 84–91. [Google Scholar] [PubMed]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sands, B.E.; Rutgeerts, P.; Sandborn, W.; Danese, S.; D’Haens, G.; Panaccione, R.; Loftus, E.V., Jr.; Sankoh, S.; Fox, I.; et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017, 66, 839–851. [Google Scholar] [CrossRef]
- Weisshof, R.; El Jurdi, K.; Zmeter, N.; Rubin, D.T. Emerging Therapies for Inflammatory Bowel Disease. Adv. Ther. 2018, 35, 1746–1762. [Google Scholar] [CrossRef] [Green Version]
- Streeter, P.R.; Berg, E.L.; Rouse, B.T.; Bargatze, R.F.; Butcher, E.C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nture 1988, 331, 41–46. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005, 353, 1912–1925. [Google Scholar] [CrossRef]
- Danese, S.; Vuitton, L.; Peyrin-Biroulet, L. Biologic agents for IBD: Practical insights. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 537–545. [Google Scholar] [CrossRef]
- Yamamoto-Furusho, J.K. Inflammatory bowel disease therapy: Blockade of cytokines and cytokine signaling pathways. Curr. Opin. Gastroenterol. 2018, 34, 187–193. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bersudsky, M.; Luski, L.; Fishman, D.; White, R.M.; Ziv-Sokolovskaya, N.; Dotan, S.; Rider, P.; Kaplanov, I.; Aychek, T.; Dinarello, C.A.; et al. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut 2014, 63, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of Immunogenicity on the Long-Term Efficacy of Infliximab in Crohn’s Disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef]
- Allez, M.; Karmiris, K.; Louis, E.; Van Assche, G.; Ben-Horin, S.; Klein, A.; Van der Woude, J.; Baert, F.; Eliakim, R.; Katsanos, K.; et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. J. Crohn’s Colitis 2010, 4, 355–366. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, C.K.; Lee, J.K.; Jeong, S.J.; Oh, S.J.; Moon, J.R.; Kim, H.S.; Kim, H.J. Long-term evolution of direct healthcare costs for inflammatory bowel diseases: A population-based study (2006–2015). Scand. J. Gastroenterol. 2019, 54, 419–426. [Google Scholar] [CrossRef]
- Mak, J.W.; Sung, J.J. The Use of Biologics and Biosimilar in Asian patients with IBD: Are we ready? J. Gastroenterol. Hepatol. 2019, 34, 1269–1270. [Google Scholar] [CrossRef] [Green Version]
- Dubinsky, M. Azathioprine, 6-Mercaptopurine in Inflammatory Bowel Disease: Pharmacology, Efficacy, and Safety. Clin. Gastroenterol. Hepatol. 2004, 2, 731–743. [Google Scholar] [CrossRef]
- Chandel, S.; Prakash, A.; Medhi, B. Current scenario in inflammatory bowel disease: Drug development prospects. Pharmacol. Rep. 2015, 67, 224–229. [Google Scholar] [CrossRef]
- Iacucci, M.; de Silva, S.; Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can. J. Gastroenterol. 2010, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.J.; Lobo, A.J.; Travis, S.P. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004, 53 (Suppl. 5), 1–16. [Google Scholar] [CrossRef]
- Bergman, R.; Parkes, M. Systematic review: The use of mesalazine in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 23, 841–855. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Tofacitinib in the treatment of ulcerative colitis: Efficacy and safety from clinical trials to real-world experience. Therap. Adv. Gastroenterol. 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Campieri, M.; Ferguson, A.; Doe, W.; Persson, T.; Nilsson, L.G. Oral budesonide is as eVective as oral prednisolone in active Crohn’s disease. Gut 1997, 41, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, M.I.; Herfarth, H. Budesonide for the treatment of ulcerative colitis. Expert Opin. Pharmacother. 2016, 17, 1549–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuenzig, M.E.; Rezaie, A.; Kaplan, G.G.; Otley, A.R.; Steinhart, A.H.; Griffiths, A.M.; Benchimol, E.I.; Seow, C.H. Budesonide for the Induction and Maintenance of Remission in Crohn’s Disease: Systematic Review and Meta-Analysis for the Cochrane Collaboration. J. Can. Assoc. Gastroenterol. 2018, 1, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Safdi, M.; Popp, J.W., Jr.; Langholff, W.; Sandborn, W.J. Infliximab for Crohn’s Disease: More Than 13 Years of Real-world Experience. Inflamm. Bowel Dis. 2018, 24, 490–501. [Google Scholar] [CrossRef]
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Melsheimer, R.; Geldhof, A.; Apaolaza, I.; Schaible, T. Remicade((R)) (infliximab): 20 years of contributions to science and medicine. Biologics 2019, 13, 139–178. [Google Scholar] [CrossRef] [Green Version]
- Guidi, L.; Pugliese, D.; Armuzzi, A. Update on the management of inflammatory bowel disease: Specific role of adalimumab. Clin. Exp. Gastroenterol. 2011, 4, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Tursi, A.; Elisei, W.; Faggiani, R.; Allegretta, L.; Valle, N.D.; Forti, G.; Franceschi, M.; Ferronato, A.; Gallina, S.; Larussa, T.; et al. Effectiveness and safety of adalimumab to treat outpatient ulcerative colitis: A real-life multicenter, observational study in primary inflammatory bowel disease centers. Medicine (Baltimore) 2018, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Felice, C. Natalizumab in Crohn’s disease: Past and future areas of applicability. Ann. Gastroenterol. 2013, 26, 189–190. [Google Scholar] [PubMed]
- Caprilli, D.G.R. Natalizumab in the treatment of Crohn’s disease. Biol. Targets Ther. 2008, 2, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Sandborn, W.J.; Abreu, M.T.; D’Haens, G.; Colombel, J.F.; Vermeire, S.; Mitchev, K.; Jamoul, C.; Fedorak, R.N.; Spehlmann, M.E.; Wolf, D.C.; et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin. Gastroenterol. Hepatol. 2010, 8, 688–695. [Google Scholar] [CrossRef]
- Schreiber, S. Certolizumab pegol for the treatment of Crohn’s disease. Therap. Adv. Gastroenterol. 2011, 4, 375–389. [Google Scholar] [CrossRef] [Green Version]
- Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Golimumab for Rheumatoid Arthritis. J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Flamant, M.; Paul, S.; Roblin, X. Golimumab for the treatment of ulcerative colitis. Expert Opin. Biol. Ther. 2017, 17, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Russi, L.; Scharl, M.; Rogler, G.; Biedermann, L. The Efficacy and Safety of Golimumab as Third- or Fourth-Line Anti-TNF Therapy in Patients with Refractory Crohn’s Disease: A Case Series. Inflamm. Intest. Dis. 2017, 2, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Scribano, M.L. Vedolizumab for inflammatory bowel disease: From randomized controlled trials to real-life evidence. World J. Gastroenterol. 2018, 24, 2457–2467. [Google Scholar] [CrossRef]
- Weaver, K.N.; Gregory, M.; Syal, G.; Hoversten, P.; Hicks, S.B.; Patel, D.; Christophi, G.; Beniwal-Patel, P.; Isaacs, K.L.; Raffals, L.; et al. Ustekinumab Is Effective for the Treatment of Crohn’s Disease of the Pouch in a Multicenter Cohort. Inflamm. Bowel Dis. 2019, 25, 767–774. [Google Scholar] [CrossRef]
- Kotze, P.G.; Ma, C.; Almutairdi, A.; Panaccione, R. Clinical utility of ustekinumab in Crohn’s disease. J. Inflamm. Res. 2018, 11, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.J.; Fedorak, R.N.; Hommes, D.W.; Sturm, A.; Baumgart, D.C.; Bressler, B.; Schreiber, S.; Mansfield, J.C.; Williams, M.; Tang, M.; et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013, 62, 1122–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeire, S.; Ghosh, S.; Panes, J.; Dahlerup, J.F.; Luegering, A.; Sirotiakova, J.; Strauch, U.; Burgess, G.; Spanton, J.; Martin, S.W.; et al. The mucosal addressin cell adhesion molecule antibody PF- 00547,659 in ulcerative colitis: A randomised study. Gut 2011, 60, 1068–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Bertani, L.; Ceccarelli, L.; Bodini, G.; Zingone, F.; Buda, A.; Facchin, S.; Lorenzon, G.; Marchi, S.; Marabotto, E.; et al. Antimicrobial treatment with the fixed-dose antibiotic combination RHB-104 for Mycobacterium avium subspecies paratuberculosis in Crohn’s disease: Pharmacological and clinical implications. Expert Opin. Biol. Ther. 2019, 19, 79–88. [Google Scholar] [CrossRef]
- Hanai, H.; Takeda, Y.; Eberhardson, M.; Gruber, R.; Saniabadi, A.R.; Winqvist, O.; Lofberg, R. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: A concise review. Clin. Exp. Immunol. 2011, 163, 50–58. [Google Scholar] [CrossRef]
- Hanai, H.; Watanabe, F.; Takeuchi, K.; Iida, T.; Yamada, M.; Iwaoka, Y.; Saniabadi, A.; Matsushita, I.; Sato, Y.; Tozawa, K.; et al. Leukocyte adsorptive apheresis for the treatment of active ulcerative colitis: A prospective, uncontrolled, pilot study. Clin. Gastroenterol. Hepatol. 2003, 1, 28–35. [Google Scholar] [CrossRef]
- Shimoyama, T.; Sawada, K.; Hiwatashi, N.; Sawada, T.; Matsueda, K.; Munakata, A.; Asakura, H.; Tanaka, T.; Kasukawa, R.; Kimura, K.; et al. Safety and efficacy of granulocyte and monocyte adsorption apheresis in patients with active ulcerative colitis: A multicenter study. J. Clin. Apher. 2001, 16, 1–9. [Google Scholar] [CrossRef]
- Dignass, A.U.; Eriksson, A.; Kilander, A.; Pukitis, A.; Rhodes, J.M.; Vavricka, S. Clinical trial: Five or ten cycles of granulocyte-monocyte apheresis show equivalent efficacy and safety in ulcerative colitis. Aliment. Pharmacol. Ther. 2010, 31, 1286–1295. [Google Scholar] [CrossRef] [Green Version]
- Bemelman, W.A.; S-ECCO collaborators. Evolving Role of IBD Surgery. J. Crohn’s Colitis 2018, 12, 1005–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, F.; Klar, E. Surgical Principles in the Treatment of Ulcerative Colitis. Viszeralmedizin 2015, 31, 246–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frolkis, A.D.; Dykeman, J.; Negron, M.E.; Debruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef]
- Wong, D.J.; Roth, E.M.; Feuerstein, J.D.; Poylin, V.Y. Surgery in the age of biologics. Gastroenterol. Rep. 2019, 7, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifarth, C.; Kreis, M.E.; Grone, J. Indications and Specific Surgical Techniques in Crohn’s Disease. Viszeralmedizin 2015, 31, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cima, R.R.; Pemberton, J.H. Early surgical intervention in ulcerative colitis. Gut 2004, 53, 306–307. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Lashner, B.A. Diagnosis and Treatment of Pouchitis. Gasteroenterol. Gastroenterol Hepatol. 2008, 4, 355–361. [Google Scholar]
- Martin, S.T.; Vogel, J.D. Restorative procedures in colonic crohn disease. Clin. Colon Rectal Surg. 2013, 26, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Fumery, M.; Dulai, P.S.; Meirick, P.; Farrell, A.M.; Ramamoorthy, S.; Sandborn, W.J.; Singh, S. Systematic review with meta-analysis: Recurrence of Crohn’s disease after total colectomy with permanent ileostomy. Aliment. Pharmacol. Ther. 2017, 45, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Bemelman, W.A.; Warusavitarne, J.; Sampietro, G.M.; Serclova, Z.; Zmora, O.; Luglio, G.; de Buck van Overstraeten, A.; Burke, J.P.; Buskens, C.J.; Colombo, F.; et al. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Ahmed Ali, U.; Keus, F.; Heikens, J.T.; Bemelman, W.A.; Berdah, S.V.; Gooszen, H.G.; van Laarhoven, C.J. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer-Berjot, L.; Maggiori, L.; Birnbaum, D.; Lefevre, J.H.; Berdah, S.; Panis, Y. A total laparoscopic approach reduces the infertility rate after ileal pouch-anal anastomosis: A 2-center study. Ann. Surg. 2013, 258, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Oresland, T.; Bemelman, W.A.; Sampietro, G.M.; Spinelli, A.; Windsor, A.; Ferrante, M.; Marteau, P.; Zmora, O.; Kotze, P.G.; Espin-Basany, E.; et al. European evidence based consensus on surgery for ulcerative colitis. J. Crohn’s Colitis 2015, 9, 4–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilsden, R.J.; Meddings, J.B.; Verhoef, M.J. Complementary and alternative medicine use by patients with inflammatory bowel disease: An Internet survey. Can. J. Gastroenterol. 1999, 13, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Hilsden, R.J.; Scott, C.M.; Verhoef, M.J. Complementary Medicine Use by Patients With Inflammatory Bowel Disease. Am. J. Gastroenterol. 1998, 93, 697–701. [Google Scholar] [CrossRef]
- Langhorst, J.; Wulfert, H.; Lauche, R.; Klose, P.; Cramer, H.; Dobos, G.J.; Korzenik, J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 86–106. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210–220. [Google Scholar]
- Langmead, L.; Feakins, R.M.; Goldthorpe, S.; Holt, H.; Tsironi, E.; De Silva, A.; Jewell, D.P.; Rampton, D.S. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 739–747. [Google Scholar] [CrossRef]
- Fernandez-Banares, F.; Hinojosa, J.; Sanchez-Lombrana, J.L.; Navarro, E.; Martınez-Salmerón, J.F.; Garcıa-Pugés, A.; Gonzalez-Huix, F.; Riera, J.; Gonzalez-Lara, V.; Domınguez-Abascal, F.; et al. Randomized Clinical Trial of Plantago ovata Seeds (Dietary Fiber) as Compared With Mesalamine in Maintaining Remission in Ulcerative Colitis. Am. J. Gastroenterol. 1999, 94, 427–433. [Google Scholar] [PubMed]
- Sandborn, W.J.; Targan, S.R.; Byers, V.S.; Rutty, D.A.; Mu, H.; Zhang, X.; Tang, T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 2013, 108, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Zheng, P.; Chen, X.; Zhou, F.; He, Q.; Yang, Y. Andrographolide presents therapeutic effect on ulcerative colitis through the inhibition of IL-23/IL-17 axis. Am. J. Transl. Res. 2018, 10, 455–473. [Google Scholar]
- Sun, J.; Shen, X.; Dong, J.; Wang, H.; Zuo, L.; Zhao, J.; Zhu, W.; Li, Y.; Gong, J.; Li, J. Tripterygium wilfordii Hook F as Maintenance Treatment for Crohn’s Disease. Am. J. Med. Sci. 2015, 350, 345–351. [Google Scholar] [CrossRef]
- Kinnucan, J. Use of Medical Cannabis in Patients With Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2018, 14, 598–601. [Google Scholar]
- Little, T.J.; Shuker, D.M.; Colegrave, N.; Day, T.; Graham, A.L. The coevolution of virulence: Tolerance in perspective. PLoS Pathog. 2010, 6, e1001006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, D.E.; Urban, J.F., Jr.; Argo, C.K.; Weinstock, J.V. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000, 14, 1848–1855. [Google Scholar] [CrossRef] [Green Version]
- Summers, R.W.; Elliott, D.E.; Urban, J.F., Jr.; Thompson, R.; Weinstock, J.V. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Heylen, M.; Ruyssers, N.E.; Gielis, E.M.; Vanhomwegen, E.; Pelckmans, P.A.; Moreels, T.G.; De Man, J.G.; De Winter, B.Y. Of worms, mice and man: An overview of experimental and clinical helminth-based therapy for inflammatory bowel disease. Pharmacol. Ther. 2014, 143, 153–167. [Google Scholar] [CrossRef]
- Johnston, M.J.; MacDonald, J.A.; McKay, D.M. Parasitic helminths: A pharmacopeia of anti-inflammatory molecules. Parasitology 2009, 136, 125–147. [Google Scholar] [CrossRef] [Green Version]
- Scholmerich, J.; Fellermann, K.; Seibold, F.W.; Rogler, G.; Langhorst, J.; Howaldt, S.; Novacek, G.; Petersen, A.M.; Bachmann, O.; Matthes, H.; et al. A Randomised, Double-blind, Placebo-controlled Trial of Trichuris suis ova in Active Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 390–399. [Google Scholar] [CrossRef] [Green Version]
- Burakoff, R.; Pabby, V.; Onyewadume, L.; Odze, R.; Adackapara, C.; Wang, W.; Friedman, S.; Hamilton, M.; Korzenik, J.; Levine, J.; et al. Blood-based biomarkers used to predict disease activity in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 1132–1140. [Google Scholar] [CrossRef]
- Dieckgraefe, B.K.; Stenson, W.F.; Korzenik, J.R.; Swanson, P.E.; Harrington, C.A. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol. Genom. 2000, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burczynski, M.E.; Peterson, R.L.; Twine, N.C.; Zuberek, K.A.; Brodeur, B.J.; Casciotti, L.; Maganti, V.; Reddy, P.S.; Strahs, A.; Immermann, F.; et al. Molecular classification of Crohn’s disease and ulcerative colitis patients using transcriptional profiles in peripheral blood mononuclear cells. J. Mol. Diagn. 2006, 8, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Guo, N.J.; Tian, H.; Marohn, M.; Gearhart, S.; Bayless, T.M.; Brant, S.R.; Kwon, J.H. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagan, B.G.; Macdonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012, 10, 1–128. [Google Scholar] [CrossRef]
- Porro, G.B.; Cassinotti, A.; Ferrara, E.; Maconi, G.; Ardizzone, S. Review article: The management of steroid dependency in ulcerative colitis. Aliment. Pharmacol. Ther. 2007, 26, 779–794. [Google Scholar] [CrossRef]
- Rufo, P.A.; Bousvaros, A. Current Therapy of Inflammatory Bowel Disease in Children. Pediatr. Drugs 2006, 8, 279–302. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Sartor, R.B.; Silverberg, M.S.; Petersson, J.; Rutgeerts, P. Future directions in inflammatory bowel disease management. J. Crohn’s Colitis 2014, 8, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasche, C.S.; Brynskov, J.; D’Haens, J.; Hanauer, S.B.; Irvine, E.J.; Jewell, D.P.; Rlachmilewitz, D.; Sachar, D.B.; Sandborn, W.J. A Simple Classification of CI rohn’s Disease: Report of the Working -Party for the World-congresses of Gastroenterology, Vienna 1998. Inflamm. Bowel Dis. 2000, 6, 8–15. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19, 5–36. [Google Scholar] [CrossRef]
- Dassopoulos, T.; Nguyen, G.C.; Bitton, A.; Bromfield, G.P.; Schumm, L.P.; Wu, Y.; Elkadri, A.; Regueiro, M.; Siemanowski, B.; Torres, E.A.; et al. Assessment of reliability and validity of IBD phenotyping within the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) IBD Genetics Consortium (IBDGC). Inflamm. Bowel Dis. 2007, 13, 975–983. [Google Scholar] [CrossRef]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, C.; Dunn, W.; Broadhurst, D.; Brown, M.; Goodacre, R.; Campbell, S.; Makin, A.; Newman, W.; Watson, A.J. T1259 Serum Metabolite Profiles Differentiate Crohn’s Disease From Ulcerative Colitis and From Healthy Controls. Gastroenterology 2010, 138, S-523. [Google Scholar] [CrossRef]
- Kolho, K.L.; Pessia, A.; Jaakkola, T.; de Vos, W.M.; Velagapudi, V. Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bjerrum, J.T.; Nielsen, O.H.; Hao, F.; Tang, H.; Nicholson, J.K.; Wang, Y.; Olsen, J. Metabonomics in Ulcerative Colitis: Diagnostics, Biomarker Identification, And Insight into the Pathophysiology. J. Proteome Res. 2010, 9, 954–962. [Google Scholar] [CrossRef]
- Nash, P.T.; Florin, T.H. Tumour necrosis factor inhibitors. J. Med. J. Austr. 2005, 183, 205–208. [Google Scholar] [CrossRef]
- Hamlin, P.J.; Warren, L.; Everett, S.M. Establishing a biologics service for patients with inflammatory bowel disease. Frontline Gastroenterol. 2011, 2, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, A.W.; Hlavka, J.P.; Case, S.R. Biosimilar cost savings in the United States: Initial experience and future potential. Rand Health Q. 2017, 7, 3. [Google Scholar]
- Kochar, B.; Barnes, E.L.; Long, M.D.; Cushing, K.C.; Galanko, J.; Martin, C.F.; Raffals, L.E.; Sandler, R.S. Depression Is Associated With More Aggressive Inflammatory Bowel Disease. Am. J. Gastroenterol. 2018, 113, 80–85. [Google Scholar] [CrossRef]
- Crohn’s & Colitis Australia. Final Report of the First Audit of the Organisation and Provision of IBD Services in Australia; Crohn’s & Colitis Australia: Camberwell, UK, 2016. [Google Scholar]
- Knowles, S.; Andrews, J.M.; Porter, A. Predictors of Impaired Mental Health and Support Seeking in Adults with Inflammatory Bowel Disease: An Online Survey. Gastroenterol. Nurs. 2018, 41, 38–46. [Google Scholar] [CrossRef]
- Massuger, W.; Moore, G.T.; Andrews, J.M.; Kilkenny, M.F.; Reyneke, M.; Knowles, S.; Purcell, L.; Alex, G.; Buckton, S.; Page, A.T.; et al. The Crohn’s & Colitis Australia inflammatory bowel disease audit: Measuring the quality of care in Australia. Intern. Med. J. 2019, 49, 859–866. [Google Scholar] [PubMed]
- Nigro, G.; Angelini, G.; Grosso, S.B.; Caula, G.; Sategna-Guidetti, C. Psychiatric Predictors of Noncompliance in Inflammatory Bowel Disease Psychiatry and Compliance. J. Clin. Gastroenterol. 2001, 32, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Tulp, M.; Bohlin, L. Functional versus chemical diversity: Is biodiversity important for drug discovery? Trends Pharmacol. Sci. 2002, 23, 225–231. [Google Scholar] [CrossRef]
- Grabowski, K.; Baringhaus, K.H.; Schneider, G. Scaffold diversity of natural products: Inspiration for combinatorial library design. Nat. Prod. Rep. 2008, 25, 892–904. [Google Scholar] [CrossRef]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef]
- Wangchuk, P.; Loukas, A. Techniques and Technologies for the Biodiscovery of Novel Small Molecule Drug Lead Compounds From Natural Products. In Natural Products and Drug Discovery: An Integrated Approach; Mandal, S., Mandal, V., Konishi, T., Eds.; Elsevier: London, UK, 2018; pp. 435–465. [Google Scholar] [CrossRef]
- Macielag, M.J. Chemical Properties of Antimicrobials and Their Uniqueness. In Antibiotic Discovery and Development; Dougherty, T.J., Pucci, M.J., Eds.; Springer: Boston, MA, USA, 2012; pp. 793–820. [Google Scholar]
- Wang, K.; Xiao, J.; Liu, X.; Jiang, Z.; Zhan, Y.; Yin, T.; He, L.; Zhang, F.; Xing, S.; Chen, B.; et al. AICD: An integrated anti-inflammatory compounds database for drug discovery. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Aswad, M.; Rayan, M.; Abu-Lafi, S.; Falah, M.; Raiyn, J.; Abdallah, Z.; Rayan, A. Nature is the best source of anti-inflammatory drugs: Indexing natural products for their anti-inflammatory bioactivity. Inflamm. Res. 2018, 67, 67–75. [Google Scholar] [CrossRef]
- Shiomi, Y.; Nishiumi, S.; Ooi, M.; Hatano, N.; Shinohara, M.; Yoshie, T.; Kondo, Y.; Furumatsu, K.; Shiomi, H.; Kutsumi, H.; et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm. Bowel Dis. 2011, 17, 2261–2274. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Okamoto, S.; Hashimoto, M.; Muramatsu, T.; Andou, A.; Uo, M.; Kitazume, M.T.; Matsuoka, K.; Yajima, T.; Inoue, N.; et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE 2012, 7, e31131. [Google Scholar] [CrossRef] [Green Version]
- Goyal, N.; Rana, A.; Ahlawat, A.; Bijjem, K.R.; Kumar, P. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef]
- Mizoguchi, A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012, 105, 263–320. [Google Scholar] [CrossRef] [PubMed]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, R.E.; Ahn, S.; Nzimiro, S.; Fotie, J.; Phelps, M.A.; Cotrill, J.; Yakovich, A.J.; Sackett, D.L.; Dalton, J.T.; Werbovetz, K.A. Inhibitors of tubulin assembly identified through screening a compound library. Chem. Biol. Drug Des. 2008, 72, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.T.; Li, X.; Liang, Y.; Wang, L.J.; Huang, Y.C.; Xiao, H.T. Plant-Derived Alkaloids: The Promising Disease-Modifying Agents for Inflammatory Bowel Disease. Front. Pharmacol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S.; et al. Berberine improved experimental chronic colitis by regulating interferon-gamma- and IL-17A-producing lamina propria CD4(+) T cells through AMPK activation. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Yu, X.T.; Xu, Y.F.; Huang, Y.F.; Qu, C.; Xu, L.Q.; Su, Z.R.; Zeng, H.F.; Zheng, L.; Yi, T.G.; Li, H.L.; et al. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and Anti-Inflammatory Activities of Resveratrol through Classic Models in Mice and Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, N.; Meena, N.K.; Majumdar, I.; Paul, J. Role of Bromelain as Herbal Anti-Inflammatory Compound Using In Vitro and In Vivo Model of Colitis. J. Autoimmune Dis. 2017, 3, 1–8. [Google Scholar]
- Shepherd, C.; Giacomin, P.; Navarro, S.; Miller, C.; Loukas, A.; Wangchuk, P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J. Ethnopharmacol. 2018, 211, 17–28. [Google Scholar] [CrossRef]
- Cho, W.; Nam, J.W.; Kang, H.J.; Windono, T.; Seo, E.K.; Lee, K.T. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappaB pathway in LPS-stimulated murine macrophages. Int. Immunopharmacol. 2009, 9, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Chaninani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Xiao, Z.; Wen, X.; Luo, J.; Chen, S.; Cheng, Z.; Xiang, D.; Hu, J.; He, J. The anti-inflammatory effect of Andrographis paniculata (Burm. f.) Nees on pelvic inflammatory disease in rats through down-regulation of the NF-kappaB pathway. BMC Complement. Altern. Med. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Canales, M.; Jimenez-Rivas, R.; Canales-Martinez, M.M.; Garcia-Lopez, A.J.; Rivera-Yanez, N.; Nieto-Yanez, O.; Ledesma-Soto, Y.; Sanchez-Torres, L.E.; Rodriguez-Sosa, M.; Terrazas, L.I.; et al. Protective Effect of Amphipterygium adstringens Extract on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Mediat. Inflamm. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Choi, R.J.; Shin, E.M.; Jung, H.A.; Choi, J.S.; Kim, Y.S. Inhibitory effects of kaurenoic acid from Aralia continentalis on LPS-induced inflammatory response in RAW264.7 macrophages. Phytomedicine 2011, 18, 677–682. [Google Scholar] [CrossRef]
- Bhanu, P.K.; Kotakadi, V.S. Anti-inflammatory Effect of Basella rubra on Oxazolone-induced Colitis in Rat. Am. J. Phytomed. Clin. Ther. 2014, 2, 832–841. [Google Scholar]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Frohlich-Nowoisky, J.; Thines, E.; Poschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, J.P.; Ocampo, Y.C.; Franco, L.A. In vivo and in vitro anti-inflammatory activity of Cryptostegia grandiflora Roxb. ex R. Br. leaves. Biol. Res. 2014, 47, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, R.C.; Claudino, R.F.; Bento, A.F.; Marcon, R.; Schmidt, E.C.; Bouzon, Z.L.; Pianowski, L.F.; Calixto, J.B. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS ONE 2011, 6, e27122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, E.J.; Ahn, K.S.; Shin, E.M.; Jung, S.H.; Kim, Y.S. Inhibition of lipopolysaccharide-induced iNOS and COX-2 expression by dehydroevodiamine through suppression of NF-kappaB activation in RAW 264.7 macrophages. Life Sci. 2006, 79, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, W.; Ma, X.; Song, D. Bioassay-guided isolation of an alkaloid with antiangiogenic and antitumor activities from the extract of Fissistigma cavaleriei root. Phytomedicine 2012, 19, 301–305. [Google Scholar] [CrossRef]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- da Silva, V.C.; de Araujo, A.A.; de Souza Araujo, D.F.; Souza Lima, M.C.J.; Vasconcelos, R.C.; de Araujo Junior, R.F.; Langasnner, S.M.Z.; de Freitas Fernandes Pedrosa, M.; de Medeiros, C.; Guerra, G.C.B. Intestinal Anti-Inflammatory Activity of the Aqueous Extract from Ipomoea asarifolia in DNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2018, 19, 4016. [Google Scholar] [CrossRef] [Green Version]
- Huo, X.; Zhang, L.; Gao, L.; Guo, Y.; Zhang, L.; Li, L.; Si, J.; Cao, L. Antiinflammatory and Analgesic Activities of Ethanol Extract and Isolated Compounds from Millettia pulchra. Biol. Pharm. Bull. 2015, 38, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Burgess, K.; Rankin, N.; Weidt, S. Metabolomics. In Handbook of Pharmacogenomics and Stratified Medicine; Padmanabhan, S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 181–205. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yu, C.; Wen, X.D.; Chen, L.; Zhang, C.F.; Calway, T.; Qiu, Y.; Wang, Y.; Zhang, Z.; Anderson, S.; et al. American Ginseng Attenuates Colitis-Associated Colon Carcinogenesis in Mice: Impact on Gut Microbiota and Metabolomics. Cancer Prev. Res. (Phila) 2016, 9, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Safarpour, A.R.; Kaviyani, F.; Sepehrimanesh, M.; Ahmadi, N.; Hosseinabadi, O.K.; Tanideh, N.; Showraki, N. Antioxidant and Anti-Inflammatory Effects of Gel and Aqueous Extract of Melilotus officinalis L. in Induced Ulcerative Colitis: A Rattus norvegicus Model. Ann. Colorectal Res. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Khodayar, B.; Farzaei, M.H.; Abdolghaffari, A.H.; Bahramsoltani, R.; Baeeri, M.; Sabbagh Ziarani, F.; Mohammadi, M.; Rahimi, R.; Abdollahi, M. The Protective Effect of the Gallic Acid Against TNBS-induced Ulcerative Colitis in Rats: Role of Inflammatory Parameters. JIMC 2018, 1, 34–42. [Google Scholar]
- Toledo, T.R.; Dejani, N.N.; Monnazzi, L.G.; Kossuga, M.H.; Berlinck, R.G.; Sette, L.D.; Medeiros, A.I. Potent anti-inflammatory activity of pyrenocine A isolated from the marine-derived fungus Penicillium paxilli Ma(G)K. Mediat. Inflamm. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Amri, O.; Zekhnini, A.; Bouhaimi, A.; Tahrouch, S.; Hatimi, A. Anti-inflammatory Activity of Methanolic Extract from Pistacia atlantica Desf. Leaves. Pharmacogn. J. 2017, 10, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, N.; Zorrilla, P.; Burkard, N.; Pischel, I.; Sievers, H.; Benedek, B.; Feistel, B.; Walbroel, B.; et al. Intestinal anti-inflammatory activity of the Serpylli herba extract in experimental models of rodent colitis. J. Crohn’s Colitis 2014, 8, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Kim, Y.H.; Lee, C.S.; Lee, J.J.; Choi, I.; Pyun, K.H. Anti-inflammatory effects of Stephania tetrandra S. Moore on interleukin-6 production and experimental inflammatory disease models. Mediat. Inflamm. 1996, 5, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Sutradhar, R.K.; Rahman, A.M.; Ahmad, M.; Bachar, S.C.; Saha, A.; Guha, S.K. Bioactive Alkaloid from Sida cordifolia Linn. wit Analgesic and Anti-Inflammatory Activities. IJPT 2006, 5, 175–178. [Google Scholar]
- Yun, K.J.; Min, B.S.; Kim, J.Y.; Lee, K.T. Styraxoside A Isolated from the Stem Bark of Styrax japonica Inhibits Lipopolysaccharide-Induced Expression of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in RAW 264.7 Cells by Suppressing Nuclear Factor-kappa B Activation. Biol. Pharm. Bull. 2007, 30, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yi, M.; Ding, L.; He, S. A Review of Anti-Inflammatory Compounds from Marine Fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.B.; Jiao, F.R.; Wu, W.; Jiao, W.H.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Preussins with Inhibition of IL-6 Expression from Aspergillus flocculosus 16D-1, a Fungus Isolated from the Marine Sponge Phakellia fusca. J. Nat. Prod. 2018, 81, 2275–2281. [Google Scholar] [CrossRef]
- Niu, S.; Xie, C.L.; Xia, J.M.; Luo, Z.H.; Shao, Z.; Yang, X.W. New anti-inflammatory guaianes from the Atlantic hydrotherm-derived fungus Graphostroma sp. MCCC 3A00421. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Xie, C.-L.; Zhong, T.; Xu, W.; Luo, Z.-H.; Shao, Z.; Yang, X.-W. Sesquiterpenes from a deep-sea-derived fungus Graphostroma sp. MCCC 3A00421. Tetrahedron 2017, 73, 7267–7273. [Google Scholar] [CrossRef]
- Renner, M.K.; Jensen, P.R.; Fenical, W. Mangicols: Structures and Biosynthesis of A New Class of Sesterterpene Polyols from a Marine Fungus of the Genus Fusarium. J. Org. Chem. 2000, 65, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, M.C.; Li, Y.; Kim, E.A.; Kang, S.M.; Jeon, Y.J. Asperflavin, an Anti-Inflammatory Compound Produced by a Marine-Derived Fungus, Eurotium amstelodami. Molecules 2017, 22, 1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.; Patil, M.D.; Soni, S.; Khobragade, T.P.; Chisti, Y.; Banerjee, U.C. Production of mycophenolic acid by Penicillium brevicompactum-A comparison of two methods of optimization. Biotechnol. Rep. (Amst) 2016, 11, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.T. Cyclosporin A Production from Tolipocladium inflatum. Gen. Med. 2017, 5, 1–3. [Google Scholar] [CrossRef]
- Mehling, M.; Johnson, T.A.; Antel, J.; Kappos, L.; Bar-Or, A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology 2011, 76, 520–527. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediat. Inflamm. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Verekar, S.A.; Periyasamy, G.; Ganguli, B.N. Fungi: A Potential Source of Anti-inflammatory Compounds. In Microorganisms in Sustainable Agriculture and Biotechnology; Satyanarayana, T., Johri, B.N., Prakash, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 613–645. [Google Scholar] [CrossRef]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef] [Green Version]
- Kahl, J.; Brattig, N.; Liebau, E. The Untapped Pharmacopeic Potential of Helminths. Trends Parasitol. 2018, 34, 828–842. [Google Scholar] [CrossRef]
- Melon, A.; Wang, A.; Phan, V.; McKay, D.M. Infection with Hymenolepis diminuta is more effective than daily corticosteroids in blocking chemically induced colitis in mice. J. Biomed. Biotechnol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.I.; Blennerhasset, P.A.; Varghese, A.K.; Chowdhury, S.K.; Omsted, P.; Deng, Y.; Collins, S.M. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heylen, M.; Ruyssers, N.E.; Nullens, S.; Schramm, G.; Pelckmans, P.A.; Moreels, T.G.; De Man, J.G.; De Winter, B.Y. Treatment with egg antigens of Schistosoma mansoni ameliorates experimental colitis in mice through a colonic T-cell-dependent mechanism. Inflamm. Bowel Dis. 2015, 21, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Shepherd, C.; Constantinoiu, C.; Ryan, R.Y.M.; Kouremenos, K.A.; Becker, L.; Jones, L.; Buitrago, G.; Giacomin, P.; Wilson, D.; et al. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun. 2019, 87, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory-secretory products of hookworm and whipworm. Metabolomics 2019, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Constantinoiu, C.; Eichenberger, R.M.; Field, M.; Loukas, A. Characterization of tapeworm metabolites and their reported biological activities. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettenworth, D.; Buyse, M.; Bohm, M.; Mennigen, R.; Czorniak, I.; Kannengiesser, K.; Brzoska, T.; Luger, T.A.; Kucharzik, T.; Domschke, W.; et al. The tripeptide KdPT protects from intestinal inflammation and maintains intestinal barrier function. Am. J. Pathol. 2011, 179, 1230–1242. [Google Scholar] [CrossRef]
- Dalmasso, G.; Charrier–Hisamuddin, L.; Nguyen, H.T.; Yan, Y.; Sitaraman, S.; Merlin, D. PepT1-Mediated Tripeptide KPV Uptake Reduces Intestinal Inflammation. Gastroenterology 2008, 134, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763. [Google Scholar] [CrossRef]
- Wada, S.; Sato, K.; Ohta, R.; Wada, E.; Bou, Y.; Fujiwara, M.; Kiyono, T.; Park, E.Y.; Aoi, W.; Takagi, T.; et al. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J. Agric. Food Chem. 2013, 61, 8807–8813. [Google Scholar] [CrossRef]
- Caceres, C.C.; Bansal, P.S.; Navarro, S.; Wilson, D.; Don, L.; Giacomin, P.; Loukas, A.; Daly, N.L. An engineered cyclic peptide alleviates symptoms of inflammation in a murine model of inflammatory bowel disease. J. Biol. Chem. 2017, 292, 10288–10294. [Google Scholar] [CrossRef] [Green Version]
- Cobos, C.; Bansal, P.S.; Jones, L.; Wangchuk, P.; Wilson, D.; Loukas, A.; Daly, N.L. Engineering of an Anti-Inflammatory Peptide Based on the Disulfide-Rich Linaclotide Scaffold. Biomedicines 2018, 6, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Drug Name | Compound Class | Trade Name(S) a | FDA Approved Year | Drug Class | ROA | Half Life a | Target | Mechanism of Action | Major Side Effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| (I) Small Molecule Drugs | ||||||||||
| Azathioprine | Imidazolyl derivative of mercaptopurine | Azasan, Imuran | 1999 | Immunosuppressant | Oral | ~2 h | CD | Metabolism of azathioprine yields 6-thioguanine (6-TGn) nucleotide that inhibits lymphocyte proliferation. 6-TGN is also thought to play role in signalling lymphocyte apoptosis by inhibiting Rac1 activation in T cells. | Nausea, vomiting, leukopenia, and increased susceptibility to infection. | [141] |
| Mesalamine | 5-aminosalicylic acid derivatives | Apriso, Asacol HD, Canasa, Delzicol, Lialda, Pentasa, Rowasa, SfRowasa | 1997 | 5-Aminosalicylic acid derivative | Oral | Variable; ~ 25 h (range: 2−296 hrs) | UC, CD | Inhibits the NF-Kβ pathway, intestinal epithelial cell injury apoptosis. | Dizziness, rhinitis, sinusitis, nasopharyngitis, back pain, abdominal pain, skin rash, eructation, constipation. | [142,143,144,145] |
| Tofacitinib | Small molecule derived from n-acylpiperidines | Xeljanz, Xeljanz XR | 2012 | JAK-inhibitor | Oral | ~3−6 h | UC | Inhibits JAK family of proteins (JAK-1, 2, 3 & TYK2), while in UC, it is via inhibition of JAK-1 subsequently downregulate IL-6 and IFN-γ. | Nasopharyngitis, headache, skin rash, diarrhoea, herpes zoster infection, upper respiratory tract infection, increased creatine phosphate. | [146] |
| (II) Biologics | ||||||||||
| Budesonide | Epimeric mixture of a non-halogenated glucocorticoid, 16 alpha, 17 alpha-(22R,S)-propylmethylenedioxypregna-1,4-diene-11 beta, 21-diol-3,20-dione. | Pulmicort, Pulmicort Flehaler | 2013 for UC 2001 for CD, | Corticosteroids | Oral | 2.3 h (children) to 3.6 h (adults). | UC, CD | Respiratory infection, rhinitis, nasopharyngitis, dyspepsia, gastroenteritis, microbial infection, otic infection, and cough. | [142,147,148,149] | |
| Infliximab | Anti-TNF-α monoclonal antibody | Inflectra, Remicade, Renflexis | 1998 | Cytokines/growth factors | IV | 7 to 12 days | CD | Binds to TNF-α, thereby interfering with endogenous TNF-α activity. | Headache, abdominal pain, nausea, anaemia, antibody development, infection, upper respiratory infection, sinusitis, cough, pharyngitis. | [150,151,152] |
| Adalimumab | Anti-TNF-α monoclonal antibody | Humira, Humira Pediatric Crohn’s Start, Humira Pen, | 2002 | Cytokines/growth factors | Sub-Q | ~2 weeks | UC, CD | Binds to TNF-α and prevent from binding its receptor and inhibit subsequent inflammatory responses. | Headache, skin rash, upper respiratory tract infection, sinusitis, antibody development. | [153,154] |
| Natalizumab | Humanized IgG4k monoclonal antibody produced in murine myeloma cells. | Tysabri | 2004 | Adhesion molecules/ chemokines | IV | 3−17 days | CD | Blocks integrin (α4 subunit) association with vascular receptors, limiting adhesion and transmigration of leukocytes. | Headache, fatigue, depression, skin rash, nausea, gastroenteritis, abdominal distress, urinary tract infection, influenza, arthralgia, limb pain, back pain, upper respiratory tract infection, flu-like symptoms, peripheral edema, chest discomfort, dermatitis, menstrual disease, diarrhoea, tooth infection, dyspepsia, vaginal infection, urinary tract infection, antibody development, muscle cramp, cough, sinusitis, tonsillitis, and microbial infections. | [155,156] |
| Certolizumab pegol (CZP) | a recombinant humanized Fab′ fragment of a monoclonal antibody | Cimzia, Cimzia 200mg, Cimzia 200 mg/mL, Cimzia-200 | 2008 | Cytokines/growth factors/ immunosuppressant | Sub-Q | 14 days | CD | Selectively neutralizes TNF-α. | Upper respiratory infection, urinary tract infection, arthralgia, rash | [142,157,158] |
| Golimumab | From genetically engineered mice with human anti-TNF antibody | Simponi, Simponi Aria | 2013 | Biologic agent, TNF blocking agent | IV | 2 weeks | UC | Inhibits TNF-α activity by binding to its receptor. | Respiratory infections (nasopharyngitis), decreased neutrophils, and microbial infections. | [142,159,160,161] |
| Vedolizumab | Monoclonal antibody | Entyvio | 2014 | Biologic agent | IV | 25 days | UC, CD | Integrin antagonist; and inhibits gut specific α4β7 integrin LPAM 1. | Upper respiratory tract infection, nasopharyngitis, headache, nausea, fatigue, cough, fever, and antibody development. | [142,162] |
| Ustekinumab | Human immunoglobulin (Ig) G1 kappa monoclonal antibody. | Stelara | 2016 for CD; 2019 for UC | Cytokines/growth factor | Sub-Q | ~19 days | UC, CD | Binds to, and interferes with the proinflammatory cytokines, IL-12 and IL-23. Ustekinumab also interferes with the expression of monocyte chemotactic protein-1 (MCP-1), TNF-α, interferon-inducible protein-10, and IL-8. | Antibody development, nasopharyngitis, headache, vaginal mycosis, vulvovaginal candidiasis, erythema at injection site, and bronchitis. | [163,164] |
| Name | Plant | Disease/Condition | Target/Objective | Clinical Phase | Location(s)/Developer |
|---|---|---|---|---|---|
| Berberine | Coptis chinensis Franch | UC | Assess the safety of berberine (berberine chloride) for UC patients in clinical remission while receiving maintenance therapy with mesalamine. | Phase I | Northwestern University Chicago, Illinois, United States. Fourth Military Medical University Xi’an, Shaanxi, China. |
| Epigallocatechin-3-gallate | C. sinensis L. | Mild to moderately active UC | Determine the Safety of an oral dose of green tea extract (Polyphenon E®) as a preliminary evidence to support its efficacy in UC. | Phase II | University of Louisville Clinical Research Center Louisville, Kentucky, United States. |
| Triptolide | Tripterygium wilfordii Hook. F. | CD | Assess the effect and safety of Tripterygium Glycosides in the treatment of CD for induction remission and compare the therapeutic effect with patients who received mesalamine. | Phase II Phase III | General Surgery Institute, Jinling Hospital Nanjing, Jiangsu, China. |
| Curcumin (1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) | C. longa L. | Both UC and CD | Determine the tolerability of curcumin in pediatric IBD patients. | Phase I | Seattle Children’s Hospital Seattle, Washington, United States. |
| CD | Study the effect of curcumin combined with thiopurines in the prevention of post-operative recurrence of CD. | Phase III | University Hospital of Clermont-Ferrand (CHU), Clermont-Ferrand, France. | ||
| UC | Evaluate the efficacy of combined therapy of curcumin + 5ASA versus 5ASA alone on mild to moderate UC patients. | Phase III | Sheba Medical Center Ramat Gan, Israel. |
| Source | Isolated Compounds | Animal Models/Cell Lines | The Main Effect on Inflammation | Ref |
|---|---|---|---|---|
| A. laciniatum | 14-O-acetylneoline | TNBS-induced mice. | Protects colonic inflammation and reduces colonic IFN-γ mRNA levels. | [248] |
| Andrographis paniculata | Ethanolic extract | Pelvic inflammatory disease induced Sprague Dawley rats. | Inhibits NF-kB signal pathway. | [253] |
| Ananas comosus | Bromelain | LPS-induced human intestinal adenocarcinoma cell line (HT29 cells); DSS-induced colitis mice. | Reduces mRNA expression of proinflammatory cytokines IL-8 and TNF-α in LPS challenged HT29 cells; reduces inflammation in DSS-induced colitis mice. | [249] |
| Amphipterygium adstringens | Alcoholic extract | DSS-induced mice BALB/c mice. | Significant reduction in levels of inflammatory cytokines TNF-α, IFN-γ, and IL-1β. | [254] |
| Aralia continentalis | Kaurenoic acid (ent-kaur-16-en-19-oic acid) | LPS-induced RAW264.7 macrophages. | Significant reduction of the diameter in carrageenan-induced paw edema mice model; Suppression of the COX-2 activity. | [255] |
| Basella rubra | Methanolic extract | Oxazolone-induced rats. | Enhances recovery from colon inflammation. | [256] |
| Cinnamomum verum | Trans-cinnamaldehyde and p-cymene | THP1 monocyte-macrophage cell line TIB-202 (ATCC). | Significant reduction of the LPS-dependent IL-8 secretion in THP1 monocytes. | [257] |
| C. dubia | Capnoidine | TNBS-induced colitis mice. | Reduces colon pathology and inflammation; reduces p-IκB-α (Ser32) and p-NF-κB p65 (Ser536) levels. | [250] |
| C. heyneana | Zedoarondiol | LPS-induced macrophage cell. | Dose dependent inhibition of LPS-stimulated NO, prostaglandin E2, IL-1β, IL-6, and TNF-α in RAW 264.7 macrophage and mouse peritoneal macrophage cells. | [251] |
| Cryptostegia grandiflora | Leaf ethanol extract | 12-O -tetradecanoyl-phorbol-13-acetate (TPA) treated mice. | Reduces inflammation and MPO in ear tissue; reduces edema and leukocyte infiltration. | [258] |
| Euphorbia tirucalli | Euphol | DSS- and TNBS-induced mice. | Inhibits the levels and expression of IL-1β, CXCL1/KC, MCP-1, MIP-2, TNF-α and IL-6 in colonic tissue; reduces the expression of NOS2, VEGF, and Ki67 in colonic tissue. | [259] |
| Evodia rutaecarpa | Evodiamine, Rutaecarpine | LPS induced-RAW 267.7 cell. | Inhibits PGE2 production. | [260] |
| Evodia fructus | Dehydroevodiamine | LPS-induced RAW 264.7 macrophages cells. | Produces mark PGE2 and COX-2 inhibition via inhibiting the NF-κB activity. | [260] |
| Fissistigma oldhamii | 7′-(3′,4′-dihydroxyphenyl)-n-[(4-methoxyphenyl) ethyl] propenamide (Z23) | LPS-induced RAW 264.7 macrophages. | Exhibits mark PGE2 inhibition via suppressing the COX-2 expression. | [261] |
| Ganoderma lucidum | DMSO extract | LPS stimulation on cancer cells. | Reduces the levels of IL-6, IL-8, MMP-2, MMP-9 in breast cancer cells. | [262] |
| Ipomoea asarifolia | Aqueous extract | Dinitrobenzene sulfonic acid (DNBS)-induced colitis in mice. | Reduces MPO activity; down-regulates the gene expression of JNK1, NF-kβ-p65, and STAT3; decreases the level of TNF-α, and IL-1β, and increases IL-10. | [263] |
| Millettia pulchra | Ethanol extract and isolated compound lanceolatin B | Xylene-induced ear edema mice. | Reduces pain; inhibits NO synthesis. | [264] |
| Panax quinquefolius | Ginsenosides | azoxymethane [265]/ DSS mouse model. | Inhibits inflammatory cytokines and restores microbiome inhibited by AOM/DSS. | [266] |
| P. quinquefolius | Aqueous extract | Acetic acid-induced UC rats. | Heals colon tissues. | [267] |
| Gallic acid | TNBS-induced UC in rats. | Reduces MPO activity. | [268] | |
| Penicillium paxillin | Pyrenocine A | LPS-induced RAW 264.7 cell line. | Inhibits nitrite production and the synthesis of proinflammatory cytokines and PGE2. | [269] |
| Pistacia atlantica | Methanolic leaf extract | Carrageenan-induced mice. | Reduces hind paw edema. | [270] |
| Serpylli herba | Aqueous extract | TNBS-induced rat colitis. | Reduces the expression of proinflammatory cytokines (TNF-α, IL-1β, IFN-γ, IL-6, and IL-17), the chemokine (MCP-1), and the adhesion molecule (ICAM-1). | [271] |
| Stephania tetrandra | Methanolic extract of roots | Silica stimulated human monocytes. | Reduces the level of IL-6. | [272] |
| Sida cordifolia | 5′-Hydroxymethyl-1′- (1,2,3,9-tetrahydro-pyrrolo [2,1-b] quinazolin-1-yl)-heptan-1-one) | Carrageenan-induced rat paw edema model. | Inhibits rat paw edema. | [273] |
| Styrax japonica | Styraxosides A | RAW 264.7 Cells. | Inhibits protein the expression levels of nitric oxide synthase (NOS) and cyclooxygenase-2 (COX-2); mRNA expression levels of NOS and COX-2, TNF-α, IL-1β; inhibits DNA binding activity of NF-kB pathway. | [274] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. https://doi.org/10.3390/jcm9051273
Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. Journal of Clinical Medicine. 2020; 9(5):1273. https://doi.org/10.3390/jcm9051273
Chicago/Turabian StyleYeshi, Karma, Roland Ruscher, Luke Hunter, Norelle L. Daly, Alex Loukas, and Phurpa Wangchuk. 2020. "Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products" Journal of Clinical Medicine 9, no. 5: 1273. https://doi.org/10.3390/jcm9051273