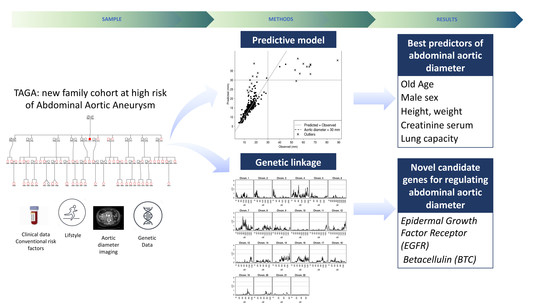
The TAGA Study: A Study of Factors Determining Aortic Diameter in Families at High Risk of Abdominal Aortic Aneurysm Reveal Two New Candidate Genes
Abstract
:1. Introduction
2. Methods
2.1. Subjects and Study Design
2.2. Clinical Conditions
2.3. Phenotype Determination
2.3.1. Ultrasound Measures
2.3.2. Pulmonary Function
2.4. Blood Collection
2.5. Genome-Wide Genotyping and Imputation
2.6. Statistical Analyses
2.6.1. Effect of Individual Phenotypes on Aortic Diameter
2.6.2. Heritabilities and Genetic Correlations
2.6.3. Multipoint Linkage Analysis
2.6.4. Fine Mapping Association Studies
2.6.5. Non-Parametric Predictive Model
3. Results
3.1. Sample Description
3.2. Association between Aortic Diameter and Related Phenotypes
3.3. Heritability and Genetic Correlations
3.4. Genome-Wide Linkage Analysis Reveals Two Significant Regions Associated with the Diameter of the Aorta
4. Discussion
4.1. Normal Variation in the Diameter of the Main Arteries is Regulated by Common Genetic Factors and are Predicted by Age, Sex, Anthropometric Measures, Pulmonary Function Tests, and Creatinine Levels
4.2. Linkage Analysis Reveals a Linkage Region Associated with Aortic Diameter on Chromosome 7
4.3. Strengths and Limitations
5. Highlights
- This study presents a new family study to understand genetic factors regulating the diameter of the aorta in individuals at high risk of AAA.
- We identify sex, age, body size, and pulmonary function as predictors of healthy aortic diameter.
- The study reveals two novel loci linked to aortic diameter, containing EGFR and BTC genes, which have previously been related to AAA, however not found in GWAS.
- The study emphasizes the importance of family studies to complement population-based GWAS studies.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| TAGA | Triple A Genomic Analysis |
| FVC | Forced vital capacity |
| FEV1 | Forced expiratory volume in 1 s |
| MAF | Minor Allele Frequency |
| IBD | Identical by descent |
| cM | Integer centimorgan |
| SNPs | Single nucleotide polymorphisms |
| GWAS | Genome-wide association study |
| COPD | Chronic obstructive pulmonary disease |
| EGFR | Endothelial Growth Factor Receptor |
References
- Pearce, W.H.; Slaughter, M.S.; LeMaire, S.; Salyapongse, A.N.; Feinglass, J.; McCarthy, W.J.; Yao, J.S.T. Aortic diameter as a function of age, gender, and body surface area. Surgery 1993, 114, 691–697. [Google Scholar] [CrossRef]
- Liddington, M.I.; Heather, B.P. The relationship between aortic diameter and body habitus. Eur. J. Vasc. Surg. 1992, 6, 89–92. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kramer-Fox, R.; O’Loughlin, J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am. J. Cardiol. 1989, 64, 507–512. [Google Scholar] [CrossRef]
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Shibamura, H.; Arthur, C.; Berguer, R.; Cole, C.W.; Juvonen, T.; Kline, R.A.; Limet, R.; MacKean, G.; Norrgård, Ö.; et al. Familial abdominal aortic aneurysms: Collection of 233 multiplex families. J. Vasc. Surg. 2003, 37, 340–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, W.H.; Zarins, C.K.; Bacharach, J.M. Atherosclerotic peripheral vascular disease symposium II controversies in abdominal aortic aneurysm repair. Proc. Circ. 2008, 118, 2860–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, P.E.; Jamrozik, K.; Lawrence-Brown, M.M.; Le, M.T.Q.; Spencer, C.A.; Tuohy, R.J.; Parsons, R.W.; Dickinson, J.A. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. Br. Med. J. 2004, 329, 1259–1262. [Google Scholar] [CrossRef] [Green Version]
- Van Der Vliet, J.A.; Boll, A.P.M. Abdominal aortic aneurysm. Lancet 1997, 349, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, G.; Zhang, J.; Duan, Z.; Xin, S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population-a meta-analysis. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Sampson, U.K.A.; Norman, P.E.; Fowkes, F.G.R.; Aboyans, V.; Song, Y.; Harrell, F.E.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M.; et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob. Heart 2014, 9, 159–170. [Google Scholar] [CrossRef]
- Pleumeekers, H.J.C.M.; Hoes, A.W.; Van Der Does, E.; Van Urk, H.; Hofman, A.; De Jong, P.T.V.M.; Grobbee, D.E. Aneurysms of the abdominal aorta in older adults: The rotterdam study. Am. J. Epidemiol. 1995, 142, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Terrin, M.C.; Dalman, R.L. Medical management of small abdominal aortic aneurysms. Circulation 2008, 117, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Clifton, M.A. Familial abdominal aortic aneurysms. Br. J. Surg. 1977, 64, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Koepsell, T. Familial Tendency for Abdominal Aortic Aneurysms. JAMA J. Am. Med. Assoc. 1986, 256, 1934–1936. [Google Scholar] [CrossRef]
- Larsson, E.; Granath, F.; Swedenborg, J.; Hultgren, R. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J. Vasc. Surg. 2009, 49, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.T.; Tromp, G.; Kuivaniemi, H.; Gretarsdottir, S.; Baas, A.F.; Giusti, B.; Strauss, E.; Van’T Hof, F.N.G.; Webb, T.R.; Erdman, R.; et al. Meta-Analysis of Genome-Wide Association Studies for Abdominal Aortic Aneurysm Identifies Four New Disease-Specific Risk Loci. Circ. Res. 2017, 120, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Fleming, C. Clinical Guidelines Screening for Abdominal Aortic Aneurysm: A Best-Evidence Systematic. Ann. Intern. Med. 2005, 142, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Almasy, L.; Blangero, J. Endophenotypes as quantitative risk factors for psychiatric disease: Rationale and study design. Am. J. Med. Genet. 2001, 105, 42–44. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284. [Google Scholar] [CrossRef] [Green Version]
- Hodge, S.E.; Vieland, V.J. The essence of single ascertainment. Genetics 1996, 144, 1215–1223. [Google Scholar]
- Ziyatdinov, A.; Brunel, H.; Martinez-Perez, A.; Buil, A.; Perera, A.; Soria, J.M. Solarius: An R interface to SOLAR for variance component analysis in pedigrees. Bioinformatics 2016, 32, 1901–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasy, L.; Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998, 62, 1198–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Abney, M. Identity by descent estimation with dense genome-wide genotype data. Genet. Epidemiol. 2011, 35, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halldorsson, B.V.; Palsson, G.; Stefansson, O.A.; Jonsson, H.; Hardarson, M.T.; Eggertsson, H.P.; Gunnarsson, B.; Oddsson, A.; Halldorsson, G.H.; Zink, F.; et al. Human genetics: Characterizing mutagenic effects of recombination through a sequence-level genetic map. Science 2019, 363, eaau1043. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef] [Green Version]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.R.; Willer, C.J.; Frishman, D. LocusZoom: Regional visualization of genome-wide association scan results. Proc. Bioinform. 2011, 27, 2336–2337. [Google Scholar] [CrossRef] [Green Version]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Tarnoki, A.D.; Tarnoki, D.L.; Littvay, L.; Garami, Z.; Karlinger, K.; Berczi, V. Genetic and environmental effects on the abdominal aortic diameter development. Arq. Bras. Cardiol. 2016, 106, 13–17. [Google Scholar] [CrossRef]
- Munk, A.; Darge, K.; Wiesel, M.; Troeger, J. Diameter of the infrarenal aorta and the iliac arteries in children: Ultrasound measurements. Transplantation 2002, 73, 631–635. [Google Scholar] [CrossRef]
- Turkbey, E.B.; Jain, A.; Johnson, C.; Redheuil, A.; Arai, A.E.; Gomes, A.S.; Carr, J.; Hundley, W.G.; Teixido-Tura, G.; Eng, J.; et al. Determinants and normal values of ascending aortic diameter by age, gender, and race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Magn. Reson. Imaging. 2014, 39, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Wolak, A.; Gransar, H.; Thomson, L.E.J.; Friedman, J.D.; Hachamovitch, R.; Gutstein, A.; Shaw, L.J.; Polk, D.; Wong, N.D.; Saouaf, R.; et al. Aortic Size Assessment by Noncontrast Cardiac Computed Tomography: Normal Limits by Age, Gender, and Body Surface Area. JACC Cardiovasc. Imaging 2008, 1, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbel, R.; Eggebrecht, H. Aortic dimensions and the risk of dissection. Heart 2006, 92, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zommorodi, S.; Leander, K.; Roy, J.; Steuer, J.; Hultgren, R. Understanding abdominal aortic aneurysm epidemiology: Socioeconomic position affects outcome. J. Epidemiol. Community Health 2018, 72, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Littooy, F.N.; Bandyk, D.; Krupski, W.C.; Barone, G.W.; Acher, C.W.; Ballard, D.J. Prevalence and associations of abdominal aortic aneurysm detected through screening. Ann. Intern. Med. 1997, 126, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Kwan, K.; DiTomasso, D.; Wright, C.M.; Criqui, M.H. The epidemiology of abdominal aortic diameter. J. Vasc. Surg. 2008, 48, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Umemoto, T. A meta-analysis of the association of chronic obstructive pulmonary disease with abdominal aortic aneurysm presence. Ann. Vasc. Surg. 2016, 34, 84–94. [Google Scholar] [CrossRef]
- Cabrera López, C.; Casanova Macario, C.; Marín Trigo, J.M.; de-Torres, J.P.; Torres, R.S.; González, J.M.; Polverino, F.; Divo, M.; Pinto Plata, V.; Zulueta, J.; et al. Prognostic Validation Using GesEPOC 2017 Severity Criteria. Arch. Bronconeumol. 2019, 55, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Siafakas, N.M.; Antoniou, K.M.; Tzortzaki, E.G. Role of angiogenesis and vascular remodeling in chronic obstructive pulmonary disease. Int. J. COPD 2007, 2, 453–462. [Google Scholar]
- Sakamaki, F.; Oya, H.; Nagaya, N.; Kyotani, S.; Satoh, T.; Nakanishi, N. Higher prevalence of obstructive airway disease in patients with thoracic or abdominal aortic aneurysm. J. Vasc. Surg. 2002, 36, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Lindholt, J.S.; Heickendorff, L.; Antonsen, S.; Fasting, H.; Henneberg, E.W. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J. Vasc. Surg. 1998, 28, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Nüesch, E.; Dale, C.; Palmer, T.M.; White, J.; Keating, B.J.; van Iperen, E.P.A.; Goel, A.; Padmanabhan, S.; Asselbergs, F.W.; Verschuren, W.M.; et al. Adult height, coronary heart disease and stroke: A multi-locus Mendelian randomization meta-analysis. Int. J. Epidemiol. 2016, 45, 1927–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.D.; Hart, C.; Upton, M.; Hole, D.; Gillis, C.; Watt, G.; Hawthorne, V. Height and risk of death among men and women: Aetiological implications of associations with cardiorespiratory disease and cancer mortality. J. Epidemiol. Community Health 2000, 54, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Schlesinger, S.; Norat, T.; Riboli, E. Tobacco smoking and the risk of abdominal aortic aneurysm: A systematic review and meta-analysis of prospective studies. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Wilmink, T.B.M.; Quick, C.R.G.; Day, N.E. The association between cigarette smoking and abdominal aortic aneurysms. J. Vasc. Surg. 1999, 30, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Stavenow, L. Stimulation of collagen secretion by factors released from injured arterial smooth muscle cells in culture. Atherosclerosis 1986, 59, 187–197. [Google Scholar] [CrossRef]
- Kalmes, A.; Daum, G.; Clowes, A.W. EGFR Transactivation in the Regulation of SMC Function. Ann. N. Y. Acad. Sci. 2006, 947, 42–55. [Google Scholar] [CrossRef]
- Ohtsu, H.; Dempsey, P.J.; Frank, G.D.; Brailoiu, E.; Higuchi, S.; Suzuki, H.; Nakashima, H.; Eguchi, K.; Eguchi, S. ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Obama, T.; Tsuji, T.; Kobayashi, T.; Fukuda, Y.; Takayanagi, T.; Taro, Y.; Kawai, T.; Forrester, S.J.; Elliott, K.J.; Choi, E.; et al. Epidermal growth factor receptor inhibitor protects against abdominal aortic aneurysm in a mouse model. Clin. Sci. 2015, 128, 559–565. [Google Scholar] [CrossRef]
- Karlsson Linnér, R.; Biroli, P.; Kong, E.; Meddens, S.F.W.; Wedow, R.; Fontana, M.A.; Lebreton, M.; Tino, S.P.; Abdellaoui, A.; Hammerschlag, A.R.; et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat. Genet. 2019, 51, 245–257. [Google Scholar] [CrossRef]
- Lauc, G.; Huffman, J.E.; Pučić, M.; Zgaga, L.; Adamczyk, B.; Mužinić, A.; Novokmet, M.; Polašek, O.; Gornik, O.; Krištić, J.; et al. Loci Associated with N-Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahola-Olli, A.V.; Würtz, P.; Havulinna, A.S.; Aalto, K.; Pitkänen, N.; Lehtimäki, T.; Kähönen, M.; Lyytikäinen, L.P.; Raitoharju, E.; Seppälä, I.; et al. Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am. J. Hum. Genet. 2017, 100, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichler, E.E.; Flint, J.; Gibson, G.; Kong, A.; Leal, S.M.; Moore, J.H.; Nadeau, J.H. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 2010, 120, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, J.; Wang, J.; Leal, S.M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 2015, 16, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.S.; Peterson, J.L.; Ceresa, B.P. Betacellulin (BTC) biases the EGFR To dimerize with ErbB. Mol. Pharmacol. 2018, 94, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era—Concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef]
- Benchek, P.H.; Morris, N.J. How meaningful are heritability estimates of liability? Hum. Genet. 2013, 132, 1351–1369. [Google Scholar] [CrossRef] [Green Version]




| Male | Female | p-Value | |
|---|---|---|---|
| n | 196 | 211 | - |
| Age (years) | 38.7 ± 22.8 | 38.4 ± 21.3 | 0.95 |
| Age range years | 2–88 | 2–85 | - |
| BMI (kg/m2) | 24.8 (5.8) | 24.3 (6.0) | 0.395 |
| Smokers | 101 (51.5) | 77 (36.5) | 0.003 |
| Dyslipidemia | 47 (24.0) | 31 (14.7) | 0.024 |
| Chronic hypertension | 37 (18.9) | 26 (12.3) | 0.091 |
| Diabetes | 12 (6.1) | 12 (5.7) | 1 |
| Neoplasia | 13 (6.6) | 13 (6.2) | 1 |
| FEV1/FVC < 0.70 | 24 (12.2) | 14 (6.6) | 0.076 |
| IHD | 5 (2.6) | 0 | 0.025 |
| BVD | 1 (0.5) | 1 (0.5) | 1 |
| AAA | 11 (5.6) | 0 | 0.001 |
| Male | Female | p-Value | Male Including AAA | |
|---|---|---|---|---|
| 0–19 years (n) | 54 | 54 | ||
| Abdominal aorta | 10.9 ± 3.0 | 10.0 ± 2.5 | 0.11 | |
| Left femoral | 6.7 ± 1.6 | 6.3 ± 1.5 | 0.19 | |
| Right femoral | 6.6 ± 1.6 | 6.1 ± 1.5 | 0.14 | |
| Left popliteal | 4.5 ± 1.2 | 4.4 ± 1.1 | 0.54 | |
| Right popliteal | 4.6 ± 1.2 | 4.3 ± 1.1 | 0.23 | |
| 20–39 years (n) | 39 | 50 | ||
| Abdominal aorta | 15.8 ± 2.2 | 13.7 ± 1.3 | <0.001 | |
| Left femoral | 9.3 ± 1.4 | 7.8 ± 0.7 | <0.001 | |
| Right femoral | 9.1 ± 1.4 | 7.7 ± 0.7 | <0.001 | |
| Left popliteal | 6.3 ± 0.8 | 5.3 ± 0.7 | <0.001 | |
| Right popliteal | 6.3 ± 0.9 | 5.4 ± 0.7 | <0.001 | |
| 40–59 years (n) | 65 | 70 | ||
| Abdominal aorta | 16.9 ± 2.0 | 15.1 ± 2.1 | <0.001 | |
| Left femoral | 9.9 ± 1.1 | 8.2 ± 1.3 | <0.001 | |
| Right femoral | 9.8 ± 1.1 | 8.3 ± 1.1 | <0.001 | |
| Left popliteal | 6.7 ± 1.0 | 5.7 ± 1.0 | <0.001 | |
| Right popliteal | 6.9 ± 1.0 | 5.7 ± 1.0 | <0.001 | |
| >60 years (n) | 27 | 37 | 27+11 | |
| Abdominal aorta | 19.6 ± 4.2 | 15.4 ± 2.5 | <0.001 | 31.4 ± 20.1 |
| Left femoral | 10.3 ± 1.6 | 8.8 ± 1.3 | <0.001 | 10.3 ± 1.5 |
| Right femoral | 10.6 ± 1.5 | 8.8 ± 1.4 | <0.001 | 10.6 ± 1.4 |
| Left popliteal | 7.7 ± 1.5 | 6.0 ± 1.0 | <0.001 | 7.4 ± 1.6 |
| Right popliteal | 7.6 ± 1.5 | 6.2 ± 1.2 | <0.001 | 7.5 ± 1.6 |
| Beta | SD | p-Value | Probands | n | |
|---|---|---|---|---|---|
| Age (years) | 0.23 | 0.01 | 1.13 × 10−52 | 12 | 407 |
| Sex (woman) | −1.81 | 0.16 | 1.86 × 10−25 | 12 | 407 |
| Left femoral major diameter (mm) | 1.29 | 0.10 | 3.1 × 10−31 | 10 | 404 |
| Right femoral major diameter (mm) | 1.19 | 0.11 | 1.5 × 10−23 | 10 | 404 |
| Left popliteal major diameter (mm) | 1.33 | 0.14 | 2.9 × 10−19 | 11 | 388 |
| Right popliteal major diameter (mm) | 1.06 | 0.14 | 7.4 × 10−14 | 11 | 404 |
| Height (cm) | 0.110 | 0.01 | 1.4 × 10−23 | 11 | 388 |
| Weight (kg) | 0.10 | 0.01 | 9.9 × 10−22 | 11 | 388 |
| FEV1 (L) | 1.66 | 0.19 | 8.1 × 10−17 | 11 | 389 |
| FVC (L) | 1.43 | 0.17 | 1.4 × 10−16 | 11 | 389 |
| Serum creatinine (mmol/L) | 0.09 | 0.01 | 5.0 × 10−10 | 11 | 367 |
| Waist circumference (cm) | 0.08 | 0.01 | 3.4 × 10−9 | 10 | 384 |
| BMI (kg/m2) | 0.14 | 0.03 | 8.2 × 10−6 | 11 | 388 |
| Mean corpuscular volume (MCV) (fL) | 0.12 | 0.03 | 6.1 × 10−4 | 11 | 367 |
| Smoker | 0.26 | 0.1 | 0.015 | 12 | 313 |
| h2 | p-Value h2 | Genetic Correlation | p-Value | Phenotypic Correlation | p-Value | Environmental Correlation | p-Value |
|---|---|---|---|---|---|---|---|
| 0.45 | 1.4 × 10−11 | 0.81 | 4.5 × 10−8 | 0.48 | 1.7 × 10−22 | 0.25 | 1.1 × 10−2 |
| 0.36 | 8.9 × 10−9 | 0.82 | 6.7 × 10−7 | 0.57 | 2.4 × 10−33 | 0.43 | 1.1 × 10−6 |
| 0.41 | 5.5 × 10−12 | 0.61 | 1.5 × 10−4 | 0.50 | 5.7 × 10−24 | 0.44 | 4.0 × 10−7 |
| 0.38 | 2.5 × 10−9 | 0.63 | 1.7 × 10−4 | 0.39 | 3.6 × 10−15 | 0.27 | 2.1 × 10−3 |
| 0.30 | 1.5 × 10−8 | 0.60 | 4.0 × 10−4 | 0.51 | 9.9 × 10−25 | 0.47 | 2.7 × 10−9 |
| 0.27 | 6.2 × 10−6 | 0.52 | 5.7 × 10−3 | 0.43 | 7.7 × 10−17 | 0.39 | 8.8 × 10−7 |
| Chr | cM | Max LOD | Closest Genes | Relevant Associated Phenotypes in the Region (GWAS Catalog) |
|---|---|---|---|---|
| 4 | 83 | 3.02 | BTC | FEV1/FVC ratio (PMID: 30804560) FEV1 (PMID:30804560) COPD (PMID:30804561) |
| 7 | 72–73 | 3.18 | EGFR POM121L12 HAUS6P1 | Smoking behavior (PMID:30643258) Serum IgG glycosylation (PMID:23382691) Circulating cytokines (PMID:27989323) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peypoch, O.; Paüls-Vergés, F.; Vázquez-Santiago, M.; Dilme, J.; Romero, J.; Giner, J.; Plaza, V.; Escudero, J.R.; Soria, J.M.; Camacho, M.; et al. The TAGA Study: A Study of Factors Determining Aortic Diameter in Families at High Risk of Abdominal Aortic Aneurysm Reveal Two New Candidate Genes. J. Clin. Med. 2020, 9, 1242. https://doi.org/10.3390/jcm9041242
Peypoch O, Paüls-Vergés F, Vázquez-Santiago M, Dilme J, Romero J, Giner J, Plaza V, Escudero JR, Soria JM, Camacho M, et al. The TAGA Study: A Study of Factors Determining Aortic Diameter in Families at High Risk of Abdominal Aortic Aneurysm Reveal Two New Candidate Genes. Journal of Clinical Medicine. 2020; 9(4):1242. https://doi.org/10.3390/jcm9041242
Chicago/Turabian StylePeypoch, Olga, Ferran Paüls-Vergés, Miquel Vázquez-Santiago, Jaime Dilme, Jose Romero, Jordi Giner, Vicente Plaza, Jose Roman Escudero, Jose Manuel Soria, Mercedes Camacho, and et al. 2020. "The TAGA Study: A Study of Factors Determining Aortic Diameter in Families at High Risk of Abdominal Aortic Aneurysm Reveal Two New Candidate Genes" Journal of Clinical Medicine 9, no. 4: 1242. https://doi.org/10.3390/jcm9041242