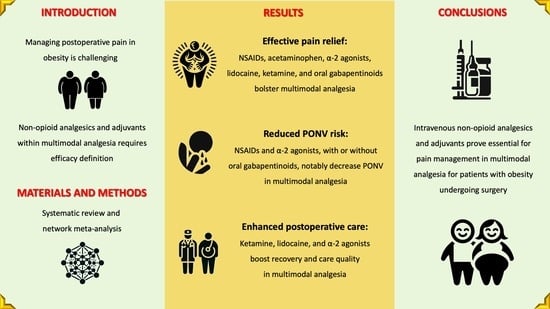
Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population (P): The population of interest includes adult patients (aged ≥ 18 years) with obesity, defined as having a Body Mass Index (BMI) of ≥30 kg/m2, who are undergoing surgery.
- Intervention (I): The interventions under evaluation include multimodal, non-opioid analgesic approaches within the context of a standard anesthesiological strategy. These include the use of non-opioid analgesics and adjuvants utilized in multimodal general anesthesia, such as acetaminophen (or paracetamol), NSAIDs, ketamine, α-2 agonists (i.e., dexmedetomidine, clonidine), lidocaine, magnesium, and oral gabapentinoids (i.e., pregabalin, gabapentin) [3]. This study will consider these interventions both individually and in various combinations.
- Comparison (C): The comparator groups in this study consist of a placebo, no intervention, or alternative multimodal analgesic strategies, employed either as single agents or in combination.
- Outcomes (O): The primary outcome of interest in this study is the level of postoperative pain, which is assessed using standardized tools such as the Visual Analogue Scale (VAS) or the Numerical Rating Scale (NRS). The VAS is typically a 10 cm line ranging from “no pain” to “worst pain imaginable”, where patients mark their pain level. The NRS, on the other hand, asks patients to rate their pain on a scale usually from 0 (“no pain”) to 10 (“worst pain possible”), allowing for a numerical assessment of their pain intensity. Both scales are widely used in clinical settings for their simplicity and effectiveness in pain evaluation. Starting from the first reported time-point for the primary outcome, evaluation was extended as long as feasible to explore potential impacts not only in the immediate but also in the late postoperative period. Secondary outcomes encompass the requirement for rescue analgesic medication, the occurrence of PONV, and the assessment of post-surgical recovery quality utilizing the Quality of Recovery-40 (QoR-40) questionnaire. The QoR-40 is a detailed survey that captures various aspects of a patient’s recovery experience following surgery and anesthesia [9].
- Study Design (S): Eligible studies for this review are prospective randomized controlled clinical trials (RCTs) published in the English language and involving adult surgical patients.
2.2. Search Strategy
2.3. Study Selection, Data Extraction and Data Retrieval
2.4. Quality Assessment and Certainty of Evidence Assessment
2.5. Statistical Analysis
3. Results
3.1. Paper Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Outcomes
3.4.1. Postoperative Pain
| VAS at the End of Surgery | τ 2 = 0.4460; τ = 0.6678; I2 = 76% [58.0%; 86.3%]; p < 0.001 at Q Test | ||||||
|---|---|---|---|---|---|---|---|
| Drug | MD | 95% CI | z | p Value | Rank | P Score | QoE |
| Ibuprofen | −3.27 | [−4.39; −2.16] | −5.79 | <0.001 | 1 | 0.954 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −2.65 | [−3.75; −1.55] | −4.74 | <0.001 | 2 | 0.838 | ⊕⊕⊕⊖ Moderate § |
| Lidocaine | −2.16 | [−3.44; −0.88] | −3.31 | <0.001 | 3 | 0.727 | ⊕⊕⊕⊖ Moderate § |
| Ketamine + clonidine | −2.00 | [−3.33; −0.66] | −2.94 | 0.003 | 4 | 0.679 | ⊕⊕⊕⊖ Moderate § |
| Acetminophen | −1.88 | [−2.84; −0.91] | −3.82 | <0.001 | 5 | 0.648 | ⊕⊕⊕⊖ Moderate § |
| Clonidine | −1.51 | [−3.37; 0.34] | −1.60 | 0.109 | 6 | 0.554 | ⊕⊕⊖⊖ Low §# |
| Ketamine | −0.68 | [−1.50; 0.13] | −1.63 | 0.103 | 7 | 0.334 | ⊕⊕⊖⊖ Low §# |
| Dexmedetomidine | −0.53 | [−1.32; 0.24] | −1.35 | 0.178 | 8 | 0.278 | ⊕⊕⊖⊖ Low §# |
| Pregabalin | −0.20 | [−1.77; 1.37] | −0.25 | 0.803 | 9 | 0.202 | ⊕⊕⊖⊖ Low §# |
| Ketamine + magnesium | −0.17 | [−1.54; 1.18] | −0.25 | 0.799 | 10 | 0.182 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 11 | 0.099 | |
| VAS 30 min | τ2 = 1.65; τ = 1.28; I2 = 88.5% [77.6%; 94.1%]; p < 0.001 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Ketamine + clonidine | −2.00 | [−4.54; 0.54] | −1.54 | 0.123 | 1 | 0.765 | ⊕⊕⊖⊖ Low §# |
| Dexmedetomidine | −1.36 | [−2.59; −0.12] | −2.15 | 0.031 | 2 | 0.639 | ⊕⊕⊕⊖ Moderate § |
| Magnesium | −1.00 | [−3.63; 1.63] | −0.74 | 0.457 | 3 | 0.498 | ⊕⊕⊖⊖ Low § |
| Ketamine | −0.89 | [−2.90; 1.10] | −0.88 | 0.380 | 4 | 0.472 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 5 | 0.124 | |
| VAS 60 min | τ2 = 1.3943; τ = 1.18; I2 = 88.7% [82.7%; 92.6%]; p < 0.001 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Lidocaine | −2.23 | [−3.61; −0.85] | −3.17 | 0.001 | 1 | 0.836 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −2.20 | [−4.59; 0.19] | −1.80 | 0.072 | 2 | 0.764 | ⊕⊕⊖⊖ Low §# |
| Ketamine | −1.31 | [−2.33; −0.29] | −2.53 | 0.011 | 3 | 0.546 | ⊕⊕⊕⊖ Moderate § |
| Dexmedetomidine | −1.24 | [−2.16; −0.33] | −2.67 | 0.007 | 4 | 0.519 | ⊕⊕⊕⊖ Moderate § |
| Ketamine + clonidine | −1.00 | [−3.34; 1.34] | −0.83 | 0.403 | 5 | 0.453 | ⊕⊕⊖⊖ Low §# |
| Pregabalin | −0.20 | [−2.93; 2.53] | −0.14 | 0.885 | 6 | 0.264 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 7 | 0.115 | |
| VAS 2 h | τ2 = 0.1162; τ = 0.3408; I2 = 50.5% [0.0%; 76.0%]; p < 0.001 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Ibuprofen | −2.37 | [−3.32; −1.41] | −4.85 | <0.001 | 1 | 0.988 | ⊕⊕⊕⊖ Moderate § |
| Acetaminophen | −1.53 | [−2.48; −0.57] | −3.16 | 0.001 | 2 | 0.785 | ⊕⊕⊕⊖ Moderate § |
| Ketamine | −1.03 | [−1.65; −0.40] | −3.23 | 0.001 | 3 | 0.624 | ⊕⊕⊕⊖ Moderate § |
| Dexmedetomidine | −0.87 | [−1.69; −0.05] | −2.10 | 0.035 | 4 | 0.545 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −0.60 | [−1.80; 0.60] | −0.98 | 0.328 | 5 | 0.418 | ⊕⊕⊖⊖ Low §# |
| Lidocaine | −0.28 | [−1.25; 0.67] | −0.59 | 0.557 | 6 | 0.278 | ⊕⊕⊖⊖ Low §# |
| Magnesium | −0.17 | [−1.32; 0.98] | −0.29 | 0.773 | 7 | 0.237 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 8 | 0.121 | |
| VAS 4 h | τ2 = 0.2421; τ = 0.4921; I2 = 59% [10.4%; 81.2%]; p < 0.001 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Dexmedetomidine | −3.10 | [−5.35; −0.84] | −2.70 | 0.006 | 1 | 0.925 | ⊕⊕⊕⊖ Moderate § |
| Magnesium | −2.06 | [−3.58; −0.53] | −2.65 | 0.008 | 2 | 0.783 | ⊕⊕⊕⊖ Moderate § |
| Ibuprofen | −1.63 | [−2.88; −0.37] | −2.54 | 0.011 | 3 | 0.688 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −1.19 | [−2.09; −0.29] | −2.59 | 0.009 | 4 | 0.540 | ⊕⊕⊕⊖ Moderate § |
| Ketamine | −1.17 | [−2.57; 0.23] | −1.63 | 0.103 | 5 | 0.537 | ⊕⊕⊖⊖ Low §# |
| Acetaminophen | −1.10 | [−2.34; 0.14] | −1.73 | 0.083 | 6 | 0.496 | ⊕⊕⊖⊖ Low §# |
| Ketamine + magnesium | −0.34 | [−1.75; 1.07] | −0.47 | 0.637 | 7 | 0.248 | ⊕⊕⊖⊖ Low §# |
| Lidocaine | 0.04 | [−1.53; 1.61] | 0.05 | 0.960 | 8 | 0.161 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 9 | 0.118 | |
| VAS 6 h | τ2 = 0; τ = 0; I2 = 0% [0.0%; 74.6%]; p = 0.568 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE ‡ |
| Lidocaine | −2.27 | [−2.92; −1.63] | −6.93 | <0.001 | 1 | 0.944 | ⊕⊕⊕⊖ Moderate § |
| Clonidine | −1.73 | [−2.98; −0.48] | −2.71 | 0.006 | 2 | 0.814 | ⊕⊕⊕⊖ Moderate § |
| Ketamine + clonidine | −1.00 | [−3.04; 1.04] | −0.96 | 0.338 | 3 | 0.571 | ⊕⊕⊖⊖ Low §# |
| Dexmedetomidine | −0.44 | [−0.75; −0.12] | −2.74 | 0.006 | 4 | 0.474 | ⊕⊕⊕⊖ Moderate § |
| Ketamine | −0.36 | [−0.77; 0.04] | −1.75 | 0.080 | 5 | 0.395 | ⊕⊕⊖⊖ Low §# |
| Gabapentin | −0.20 | [−0.40; 0.01] | −1.88 | 0.060 | 6 | 0.258 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 7 | 0.041 | |
| VAS 8 h | τ2 = 0; τ = 0; I2 = 0% [10.4%; 89.6%]; p = 1.000 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE ‡ |
| Ibuprofen | −2.50 | [−2.87; −2.12] | −13.16 | <0.001 | 1 | 0.926 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −2.44 | [−3.17; −1.70] | −6.50 | <0.001 | 2 | 0.896 | ⊕⊕⊕⊖ Moderate § |
| Acetaminophen | −1.80 | [−2.19; −1.40] | −8.96 | <0.001 | 3 | 0.677 | ⊕⊕⊕⊖ Moderate § |
| Ketamine | −0.34 | [−1.17; 0.49] | −0.80 | 0.423 | 4 | 0.426 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 5 | 0.284 | |
| Ketamine + magnesium | −0.00 | [−0.84; 0.84] | −0.00 | 1.000 | 6 | 0.274 | ⊕⊕⊖⊖ Low §# |
| Magnesium | 0.77 | [0.23; 1.30] | 2.81 | 0.005 | 7 | 0.013 | ⊕⊕⊖⊖ Low §# |
| VAS 12 h | τ2 = 0.1162; τ = 0.3408; I2 = 50.5% [0.0%; 76.0%]; p = 0.033 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Ibuprofen | −2.23 | [−2.87; −1.58] | −6.79 | <0.001 | 1 | 0.964 | ⊕⊕⊕⊖ Moderate § |
| Lidocaine | −1.90 | [−2.72; −1.08] | −4.55 | <0.001 | 2 | 0.878 | ⊕⊕⊕⊖ Moderate § |
| Acetaminophen | −1.63 | [−2.27; −0.98] | −4.93 | <0.001 | 3 | 0.801 | ⊕⊕⊕⊖ Moderate § |
| Clonidine | −0.77 | [−2.00; 0.44] | −1.25 | 0.212 | 4 | 0.580 | ⊕⊕⊖⊖ Low §# |
| Ketamine | −0.43 | [−0.85; −0.00] | −1.97 | 0.048 | 5 | 0.502 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | −0.30 | [−1.11; 0.51] | −0.72 | 0.468 | 6 | 0.411 | ⊕⊕⊖⊖ Low §# |
| Dexmedetomidine | −0.19 | [−0.71; 0.31] | −0.76 | 0.450 | 7 | 0.354 | ⊕⊕⊖⊖ Low §# |
| Ketamine + clonidine | 0.00 | [−0.76; 0.76] | 0.00 | 1.000 | 8 | 0.268 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 9 | 0.231 | |
| Ketamine + magnesium | 1.06 | [0.20; 1.92] | 2.43 | 0.015 | 10 | 0.007 | ⊕⊕⊖⊖ Low §# |
| VAS 24 h | τ2 = 0.1535; τ = 0.3918; I2 = 61.8% [35.4%; 77.4%]; p < 0.001 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Ibuprofen | −1.00 | [−1.57; −0.43] | −3.45 | <0.001 | 1 | 0.919 | ⊕⊕⊕⊖ Moderate § |
| Pregabalin | −0.80 | [−1.94; 0.34] | −1.37 | 0.171 | 2 | 0.771 | ⊕⊕⊖⊖ Low §# |
| Acetaminophen | −0.45 | [−0.91; 0.03] | −1.95 | 0.051 | 3 | 0.649 | ⊕⊕⊖⊖ Low §# |
| Clonidine | −0.39 | [−1.73; 0.94] | −0.57 | 0.566 | 4 | 0.564 | ⊕⊕⊖⊖ Low §# |
| Ketamine | −0.28 | [−0.70; 0.12] | −1.36 | 0.173 | 5 | 0.543 | ⊕⊕⊖⊖ Low §# |
| Gabapentin | −0.12 | [−1.01; 0.77] | −0.26 | 0.792 | 6 | 0.409 | ⊕⊕⊖⊖ Low §# |
| Dexmedetomidine | −0.12 | [−0.64; 0.39] | −0.46 | 0.646 | 7 | 0.393 | ⊕⊕⊖⊖ Low §# |
| Lidocaine | 0.10 | [−0.93; 1.13] | 0.19 | 0.849 | 8 | 0.291 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 9 | 0.278 | |
| Ketamine + magnesium | 0.28 | [−0.62; 1.19] | 0.62 | 0.536 | 10 | 0.179 | ⊕⊕⊖⊖ Low §# |
| VAS 48 h | τ2 = 0.2421; τ = 0.4921; I2 = 59% [10.4%; 81.2%]; p = 0.017 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Dexmedetomidine | −0.80 | [−1.58; −0.02] | −2.03 | 0.042 | 1 | 0.771 | ⊕⊕⊕⊖ Moderate § |
| Lidocaine | −0.65 | [−1.40; 0.10] | −1.69 | 0.090 | 2 | 0.650 | ⊕⊕⊖⊖ Low §# |
| Acetaminophen | −0.60 | [−1.90; 0.70] | −0.90 | 0.366 | 3 | 0.575 | ⊕⊕⊖⊖ Low §# |
| Ketamine | −0.38 | [−0.99; 0.23] | −1.21 | 0.224 | 4 | 0.411 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 5 | 0.090 | |
| VAS POD7 | τ2 = 0.6176; τ = 0.7859; I2 = 67.6% [0.0%; 90.6%]; p = 0.045 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Dexmedetomidine | −1.05 | [−2.13; 0.02] | −1.91 | 0.056 | 1 | 0.915 | ⊕⊕⊖⊖ Low §# |
| Ketamine | 0.00 | [−1.57; 1.57] | 0.00 | 1.000 | 2 | 0.320 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 3 | 0.264 | |
3.4.2. Use of Rescue Analgesics
| Rescue Therapy at PACU | τ2 = NA; τ = NA; I2 NA | ||||||
|---|---|---|---|---|---|---|---|
| Drug | OR | 95% CI | z | p Value | Rank | P Score | QoE ‡ |
| Placebo | - | - | - | - | 1 | 0.784 | - |
| Pregabalin | 1.50 | [0.53; 4.17] | 0.78 | 0.437 | 2 | 0.516 | ⊕⊕⊖⊖ Low §# |
| Ibuprofen | 2.29 | [0.18; 27.80] | 0.65 | 0.024 | 2 | 0.449 | ⊕⊕⊕⊖ Moderate § |
| Acetaminophen | 3.04 | [0.30; 30.12] | 0.95 | 0.340 | 1 | 0.248 | ⊕⊕⊖⊖ Low §# |
| Rescue therapy within 6 h | τ2 = NA; τ = NA; I2 NA | ||||||
| Drug | OR | 95% CI | z | p value | Rank | P score | QoE‡ |
| Gabapentin | 0.34 | [0.11; 1.05] | −1.87 | 0.062 | - | - | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | - | - | - |
| Rescue therapy within 24 h | τ2 = 0; τ = 0; I2 0%; p = 1.000 at Q test | ||||||
| Drug | OR | 95% CI | z | p value | Rank | P score | QoE |
| Ibuprofen | 0.34 | [0.01; 8.58] | −0.65 | 0.516 | 1 | 0.711 | ⊕⊕⊕⊖ Moderate # |
| Lidocaine | 0.60 | [0.22; 1.64] | −0.99 | 0.323 | 2 | 0.673 | ⊕⊕⊕⊖ Moderate # |
| Pregabalin | 0.55 | [0.12; 2.56] | −0.75 | 0.451 | 3 | 0.670 | ⊕⊕⊕⊖ Moderate # |
| Ketamine + magnesium | 0.75 | [0.21; 2.61] | −0.45 | 0.651 | 4 | 0.575 | ⊕⊕⊕⊖ Moderate # |
| Placebo | - | - | - | - | 5 | 0.419 | - |
| Ketamine | 1.41 | [0.38; 5.26] | 0.52 | 0.603 | 6 | 0.264 | ⊕⊕⊕⊖ Moderate # |
| Acetaminophen | 1.63 | [0.63; 4.20] | 1.02 | 0.309 | 7 | 0.185 | ⊕⊕⊕⊖ Moderate # |
| Rescue therapy within 48 h | τ2 = 0.801; τ = 0.895; I2 = 51.2% [0.0%; 85.9%]; p = 0.128 at Q test | ||||||
| Drug | OR | 95% CI | z | p value | Rank | P score | QoE |
| Ketamine | 0.15 | [0.01; 1.68] | −1.53 | 0.126 | 1 | 0.879 | ⊕⊕⊖⊖ Low §# |
| Lidocaine | 0.49 | [0.02; 9.81] | −0.46 | 0.643 | 2 | 0.602 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 3 | 0.436 | - |
| Dexmedetomidine | 2.82 | [0.68; 11.65] | 1.44 | 0.150 | 4 | 0.082 | ⊕⊕⊖⊖ Low §# |
| PONV | τ2 = 0.255; τ = 0.505; I2 = 44.1% [11.8%; 64.5%; p = 0.008 at Q test | ||||||
| Drug | OR | 95% CI | z | p value | Rank | P score | QoE |
| Pregabalin + dexmedetomidine | 0.06 | [0.00; 0.72] | −2.23 | 0.025 | 1 | 0.918 | ⊕⊕⊕⊖ Moderate § |
| Clonidine | 0.16 | [0.03; 0.79] | −2.26 | 0.024 | 2 | 0.822 | ⊕⊕⊕⊖ Moderate § |
| Dexmedetomidine | 0.30 | [0.18; 0.50] | −4.53 | <0.001 | 3 | 0.707 | ⊕⊕⊕⊖ Moderate § |
| Ibuprofen | 0.32 | [0.11; 0.91] | −2.14 | 0.032 | 4 | 0.662 | ⊕⊕⊕⊖ Moderate § |
| Gabapentin | 0.33 | [0.12; 0.91] | −2.13 | 0.033 | 5 | 0.648 | ⊕⊕⊕⊖ Moderate § |
| Magnesium | 0.39 | [0.13; 1.10] | −1.70 | 0.077 | 6 | 0.582 | ⊕⊕⊖⊖ Low §# |
| Lidocaine | 0.63 | [0.63; 1.07] | −1.68 | 0.093 | 7 | 0.375 | ⊕⊕⊖⊖ Low §# |
| Ketamine | 0.77 | [0.42; 1.43] | −0.80 | 0.421 | 8 | 0.271 | ⊕⊕⊖⊖ Low §# |
| Acetaminophen | 0.82 | [0.36; 1.85] | −0.47 | 0.641 | 9 | 0.242 | ⊕⊕⊖⊖ Low §# |
| Pregabalin | 1.10 | [0.40; 2.99] | 0.20 | 0.845 | 10 | 0.142 | ⊕⊕⊖⊖ Low §# |
| Placebo | - | - | - | - | 11 | 0.125 | - |
| QoR40 POD1 | τ2 = 0; τ = 0; I2 = 0%; p = 0.539 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Placebo | - | - | - | - | 1 | 0.905 | - |
| Pregabalin | 1.60 | [−3.76; 6.96] | 0.58 | 0.559 | 2 | 0.743 | ⊕⊕⊕⊖ Moderate # |
| Ketamine | 8.66 | [2.41; 14.90] | 2.72 | 0.006 | 3 | 0.229 | ⊕⊕⊕⊕ High |
| Lidocaine | 9.88 | [7.18; 12.59] | 7.16 | <0.001 | 4 | 1.121 | ⊕⊕⊕⊕ High |
| QoR40 POD 3 | τ2 = 0; τ = 0; I2 = 0%; p = 1.000 at Q test | ||||||
| Drug | MD | 95% CI | z | p value | Rank | P score | QoE |
| Placebo | - | - | - | - | 1 | 1.000 | - |
| Lidocaine | 33.00 | [31.24; 34.75] | 36.96 | <0.001 | 2 | 0.500 | ⊕⊕⊕⊕ High |
| Dexmedetomidine | 46.00 | [44.47; 47.52] | 59 | 0 | 3 | 0.000 | ⊕⊕⊕⊕ High |
3.4.3. PONV
3.4.4. Quality of Recovery-40 (QoR-40)
4. Discussion
The Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carron, M.; Safaee Fakhr, B.; Ieppariello, G.; Foletto, M. Perioperative care of the obese patient. Br. J. Surg. 2020, 107, e39–e55. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, I.; Eipe, N. Perioperative Pain Management in Morbid Obesity. Drugs 2019, 79, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.N.; Pavone, K.J.; Naranjo, M. Multimodal General Anesthesia: Theory and Practice. Anesth. Analg. 2018, 127, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [PubMed]
- Stenberg, E.; Dos Reis Falcão, L.F.; O’Kane, M.; Liem, R.; Pournaras, D.J.; Salminen, P.; Urman, R.D.; Wadhwa, A.; Gustafsson, U.O.; Thorell, A. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations: A 2021 Update. World J. Surg. 2022, 46, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Marinari, G.; Foletto, M.; Nagliati, C.; Navarra, G.; Borrelli, V.; Bruni, V.; Fantola, G.; Moroni, R.; Tritapepe, L.; Monzani, R.; et al. Enhanced recovery after bariatric surgery: An Italian consensus statement. Surg. Endosc. 2022, 36, 7171–7186. [Google Scholar] [CrossRef]
- De Cassai, A.; Paganini, G.; Pettenuzzo, T.; Zarantonello, F.; Boscolo, A.; Tulgar, S.; Carron, M.; Munari, M.; Navalesi, P. Single-Shot Regional Anesthesia for Bariatric Surgery: A Systematic Review and Network Meta-Analysis. Obes. Surg. 2023, 33, 2687–2694. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Loannidis, J.P.A.; Clarke, M.; Devereaus, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Myles, P.S.; Weitkamp, B.; Jones, K.; Melick, J.; Hensen, S. Validity and reliability of a postoperative quality of recovery score: The QoR-40. Br. J. Anaesth. 2000, 84, 11–15. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Bakhamees, H.S.; El-Halafawy, Y.M.; El-Kerdawy, H.M.; Gouda, N.M.; Altemyatt, S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East. J. Anaesthesiol. 2007, 19, 537–551. [Google Scholar] [PubMed]
- Kamal, H.M. Ketamine as an Adjuvant to Morphine for Patient Controlled Analgesia in Morbidly Obese Patients. J. Med. Sci. 2008, 8, 364–370. [Google Scholar] [CrossRef]
- Tufanogullari, B.; White, P.F.; Peixoto, M.P.; Kianpour, D.; Lacour, T.; Griffin, J.; Skrivanek, G.; Macaluso, A.; Shah, M.; Provost, D.A. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth. Analg. 2008, 106, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Sollazzi, L.; Modesti, C.; Vitale, F.; Sacco, T.; Ciocchetti, P.; Idra, A.S.; Tacchino, R.M.; Perilli, V. Preinductive use of clonidine and ketamine improves recovery and reduces postoperative pain after bariatric surgery. Surg. Obes. Relat. Dis. 2009, 5, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Schulmeyer, M.C.; de la Maza, J.; Ovalle, C.; Farias, C.; Vives, I. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes. Surg. 2010, 20, 1678–1681. [Google Scholar] [CrossRef]
- Hasanein, R.; El-Sayed, W.; Nabil, N.; Elsayed, G. The effect of combined remifentanil and low dose ketamine infusion in patients undergoing laparoscopic gastric bypass. Egypt. J. Anaesth. 2011, 27, 255–260. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Duncan, K.; Fitzgerald, P.; Nader, A.; Gould, R.W.; McCarthy, R.J. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: A randomized double-blinded placebo-controlled trial. Obes. Surg. 2014, 24, 212–218. [Google Scholar] [CrossRef]
- Naja, Z.M.; Khatib, R.; Ziade, F.M.; Moussa, G.; Naja, Z.Z.; Naja, A.S.; Kanawati, S. Effect of clonidine versus dexmedetomidine on pain control after laparoscopic gastric sleeve: A prospective, randomized, double-blinded study. Saudi J. Anaesth. 2014, 8, S57–S62. [Google Scholar] [CrossRef]
- Hassani, V.; Pazouki, A.; Nikoubakht, N.; Chaichian, S.; Sayarifard, A.; Shakib Khankandi, A. The effect of gabapentin on reducing pain after laparoscopic gastric bypass surgery in patients with morbid obesity: A randomized clinical trial. Anesth. Pain. Med. 2015, 5, e22372. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.K.; Abdallah, N.M. Multimodal analgesia with pregabalin and dexmedetomidine in morbidly obese patients undergoing laparoscopic sleeve gastrectomy: A prospective randomized double blind placebo controlled study. Egypt. J. Anaesth. 2016, 32, 293–298. [Google Scholar] [CrossRef]
- El Chaar, M.; Stoltzfus, J.; Claros, L.; Wasylik, T. IV Acetaminophen Results in Lower Hospital Costs and Emergency Room Visits following Bariatric Surgery: A Double-Blind, Prospective, Randomized Trial in a Single Accredited Bariatric Center. J. Gastrointest. Surg. 2016, 20, 715–724. [Google Scholar] [CrossRef]
- Sherif, A.A.; Elsersy, H.E. The impact of dexmedetomidine or xylocaine continuous infusion on opioid consumption and recovery after laparoscopic sleeve gastrectomy. Minerva Anestesiol. 2017, 83, 1274–1282. [Google Scholar] [CrossRef]
- Cooke, F.E.; Samuels, J.D.; Pomp, A.; Gadalla, F.; Wu, X.; Afaneh, C.; Dakin, G.F.; Goldstein, P.A. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Acetaminophen on Hospital Length of Stay in Obese Individuals Undergoing Sleeve Gastrectomy. Obes. Surg. 2018, 28, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Erdogan Kayhan, G.; Sanli, M.; Ozgul, U.; Kirteke, R.; Yologlu, S. Comparison of intravenous ibuprofen and acetaminophen for postoperative multimodal pain management in bariatric surgery: A randomized controlled trial. J. Clin. Anesth. 2018, 50, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Lee, C.W.; Knisely, T.; Perla, S.; Barber, K.; Kia, M. Efficacy of Intravenous Acetaminophen in Length of Stay and Postoperative Pain Control in Laparoscopic Roux-en-Y Gastric Bypass Surgery Patients. Bariatr. Surg. Pract. Patient Care. 2018, 13, 103–108. [Google Scholar] [CrossRef]
- Martins, M.J.; Martins, C.P.M.O.; Castro-Alves, L.J.; Jesus, G.N.; Campos, G.O.; Sacramento, B.B.C.; Borges, L.F.; Mello, C.A.B.; Alves, R.L.; Módolo, N.S.P. Pregabalin to improve postoperative recovery in bariatric surgery: A parallel, randomized, double-blinded, placebo-controlled study. J. Pain. Res. 2018, 11, 2407–2415. [Google Scholar] [CrossRef]
- Mostafa, R.H.; Ibrahim, M.I.; Ayoub, M.H. Effect of perioperative dexmedetomidine infusion on blood glucose levels in non-diabetic morbid obese patients undergoing laparoscopic bariatric surgery. Egypt. J. Anaesth. 2018, 34, 75–81. [Google Scholar] [CrossRef]
- Rupniewska-Ladyko, A.; Malec-Milewska, M.; Kraszewska, E.; Pirozynski, M. Gabapentin before laparoscopic sleeve gastrectomy reduces postoperative oxycodone consumption in obese patients: A randomized double-blind placebo-controlled trial. Minerva Anestesiol. 2018, 84, 565–571. [Google Scholar] [CrossRef]
- Ciftci, B.; Ekinci, M.; Celik, E.C.; Kaciroglu, A.; Karakaya, M.A.; Demiraran, Y.; Ozdenkaya, Y. Comparison of Intravenous Ibuprofen and Paracetamol for Postoperative Pain Management after Laparoscopic Sleeve Gastrectomy. A Randomized Controlled Study. Obes. Surg. 2019, 29, 765–770. [Google Scholar] [CrossRef]
- El Mourad, M.B.; Arafa, S.K. Effect of intravenous versus intraperitoneal magnesium sulfate on hemodynamic parameters and postoperative analgesia during laparoscopic sleeve gastrectomy—A prospective randomized study. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 242–247. [Google Scholar] [CrossRef]
- Khan, M.U.; Bamehriz, F.Y.; Aqil, M.; Dammas, F.A.; Fadin, A.; Khokhar, R.S. The Effect of Gabapentin on Postoperative Pain, Morphine Sparing Effect and Preoperative Anxiety in Patients Going for Sleeve Gastrectomy Surgical Procedure. J. Coll. Physicians Surg. Pak. 2019, 29, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Ritchie, M.K.; Ellison, M.B.; Petrone, A.; Heiraty, P.; Tabone, L.E. A randomized control trial using intraoperative dexmedetomidine during Roux-en-Y gastric bypass surgery to reduce postoperative pain and narcotic use. Surg. Obes. Relat. Dis. 2019, 15, 588–594. [Google Scholar] [CrossRef]
- Wang, J.; Echevarria, G.C.; Doan, L.; Ekasumara, N.; Calvino, S.; Chae, F.; Martinez, E.; Robinson, E.; Cuff, G.; Franco, L.; et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: A randomised double-blind placebo controlled study. Eur. J. Anaesthesiol. 2019, 36, 16–24. [Google Scholar] [CrossRef]
- de Oliveira, C.M.B.; Coelho, L.M.G.; Valadão, J.A.; Moura, E.C.R.; da Silva, A.A.M.; de Lima, R.C.; Brunialti, M.K.C.; Salomão, R.; da Cunha Leal, P.; Sakata, R.K. Assessment of the Effect of Perioperative Venous Lidocaine on the Intensity of Pain and IL-6 Concentration After Laparoscopic Gastroplasty. Obes. Surg. 2020, 30, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, H.; Jabbour, K.; Abi Lutfallah, A.; Abou Zeid, H.; Nasser-Ayoub, E.; Abou Haidar, M.; Naccache, N. Magnesium and Ketamine Reduce Early Morphine Consumption After Open Bariatric Surgery: A Prospective Randomized Double-Blind Study. Obes. Surg. 2020, 30, 1452–1458. [Google Scholar] [CrossRef]
- Kasputytė, G.; Karbonskienė, A.; Macas, A.; Maleckas, A. Role of Ketamine in Multimodal Analgesia Protocol for Bariatric Surgery. Medicina 2020, 56, 96. [Google Scholar] [CrossRef]
- Sakata, R.K.; de Lima, R.C.; Valadão, J.A.; Leal, P.C.; Moura, E.C.; Cruz, V.P.; de Oliveira, C.M. Randomized, Double-Blind Study of the Effect of Intraoperative Intravenous Lidocaine on the Opioid Consumption and Criteria for Hospital Discharge After Bariatric Surgery. Obes. Surg. 2020, 30, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.D.; Thiruvenkatarajan, V.; McFadden, A.; Liu, W.M.; Mets, B.; Rogers, A. Analgesic efficacy of ketamine and magnesium after laparoscopic sleeve gastrectomy: A randomized, double-blind, placebo-controlled trial. J. Clin. Anesth. 2021, 68, 110097. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.D.; Smyth, D.; Vasilopoulos, T.; Friedman, J.; Sappenfield, J.W.; Alex, G. Ketamine infusion reduces narcotic requirements following gastric bypass surgery: A randomized controlled trial. Surg. Obes. Relat. Dis. 2021, 17, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Plass, F.; Nicolle, C.; Zamparini, M.; Al Issa, G.; Fiant, A.L.; Le Roux, Y.; Gérard, J.L.; Fischer, M.O.; Alvès, A.; Hanouz, J.L. Effect of intra-operative intravenous lidocaine on opioid consumption after bariatric surgery: A prospective, randomised, blinded, placebo-controlled study. Anaesthesia 2021, 76, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Seman, M.T.; Malan, S.H.; Buras, M.R.; Butterfield, R.J.; Harold, K.L.; Madura, J.A.; Rosenfeld, D.M.; Gorlin, A.W. Low-Dose Ketamine Infusion for Perioperative Pain Management in Patients Undergoing Laparoscopic Gastric Bypass: A Prospective Randomized Controlled Trial. Anesthesiol. Res. Pract. 2021, 2021, 5520517. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Wang, J.; Gao, X.; Wang, G. Effect of Intravenous Infusion of Lidocaine Compared with Ultrasound-Guided Transverse Abdominal Plane Block on the Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery. Drug Des. Devel Ther. 2022, 16, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Ustun, Y.B.; Turunc, E.; Ozbalci, G.S.; Dost, B.; Bilgin, S.; Koksal, E.; Kaya, C. Comparison of Ketamine, Dexmedetomidine and Lidocaine in Multimodal Analgesia Management Following Sleeve Gastrectomy Surgery: A Randomized Double-Blind Trial. J. Perianesth. Nurs. 2022, 37, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.Y.I.; Afifi, M.K.M.A.; Arida, E.A.E.; Abdelhady, M.M. Effect of magnesium levels on mean tissue perfusion during and after bariatric surgeries: A randomised control trial. Egypt. J. Anaesth. 2023, 39, 502–510. [Google Scholar] [CrossRef]
- Khalil, B.N.M.; Elderh, M.S.H.; Khaja, M.A.R.; El-Shaer, A.N.; Ali, B.E.E.H.; Taeimah, M.O.A. Perioperative use of ketamine infusion versus dexmedetomidine infusion for analgesia in obese patients undergoing bariatric surgery: A double-blinded three-armed randomized controlled trial. BMC Anesthesiol. 2023, 23, 108. [Google Scholar] [CrossRef]
- Yang, T.; Mudabbar, M.S.; Liu, B.; Xu, M.; Fu, Q. Intraoperative Esketamine Is Effective at Reducing Acute Postoperative Pain in Bariatric Surgery Patients: A Randomized Control Trial. Obes. Surg. 2023, 33, 2368–2374. [Google Scholar] [CrossRef]
- Yurttas, T.; Djurdjevic, M.; Schnider, T.W.; Filipovic, M. Analgesic efficacy of systemic lidocaine using lean body mass based dosing regime versus placebo in bariatric surgery: A prospective, randomised, double-blind, placebo-controlled, single-centre study. Br. J. Anaesth. 2023, 131, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, F.; Dang, J.; Zheng, H.; Ren, B.; Liu, C.; Zuo, R.; Wang, R.; Liu, T.; Wang, Z. Effect of Intraoperative Infusion of Esketamine on Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery: A Randomized Controlled Trial. Pain. Ther. 2023, 12, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Albarrak, A.A. Safety of Non-steroidal Anti-inflammatory Drugs as Part of Enhanced Recovery After Laparoscopic Sleeve Gastrectomy-A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Ashoorion, V.; Gmora, S.; Anvari, M.; Hong, D. Intravenous Acetaminophen Versus Placebo in Post-bariatric Surgery Multimodal Pain Management: A Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2019, 29, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Wu, S.C.; Chang, P.C.; Chen, I.W.; Hsing, C.H.; Lin, C.M.; Chen, J.Y.; Chu, C.C.; Sun, C.K. Impact of Intraoperative Ketamine on Postoperative Analgesic Requirement Following Bariatric Surgery: A Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2021, 31, 5446–5457. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.M.; Panwar, R.; Borle, A.; Mulier, J.P.; Sinha, A.; Goudra, B. Perioperative analgesic profile of dexmedetomidine infusions in morbidly obese undergoing bariatric surgery: A meta-analysis and trial sequential analysis. Surg. Obes. Relat. Dis. 2017, 13, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Malhan, R.S.; Deng, S.X.; Lee, Y.; Peachey, J.; Singh, M.; Hong, D. Benefits of dexmedetomidine on postoperative analgesia after bariatric surgery. Minerva Anestesiol. 2022, 88, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Munoz, M.C.; De Kock, M.; Forget, P. What is the place of clonidine in anesthesia? Systematic review and meta-analyses of randomized controlled trials. J. Clin. Anesth. 2017, 38, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Chang, Y.J.; Chen, I.W.; Chang, Y.P.; Chiu, S.F.; Sun, C.K. Efficacy of intraoperative intravenous lidocaine for postoperative analgesia following bariatric surgery: A meta-analysis of randomized controlled studies. Surg. Obes. Relat. Dis. 2022, 18, 135–147. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef]
- Albrecht, E.; Kirkham, K.R.; Liu, S.S.; Brull, R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: A meta-analysis. Anaesthesia 2013, 68, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Ieppariello, G.; Linassi, F.; Navalesi, P. Ketamine and Magnesium: A Successful Combination for Bariatric Surgery. Obes. Surg. 2020, 30, 4612–4614. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.C.; Wu, S.C.; Chiang, M.H.; Hsu, C.W.; Chen, J.Y.; Huang, P.W.; Sun, C.K. Analgesic Efficacy of Gabapentin and Pregabalin in Patients Undergoing Laparoscopic Bariatric Surgeries: A Systematic Review and Meta-analysis. Obes. Surg. 2022, 32, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.C.; Schumann, R.; Gallagher, S.; McNicol, E.D. Single-dose intravenous ibuprofen for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2021, 9, CD013264. [Google Scholar] [CrossRef]
- McNicol, E.D.; Ferguson, M.C.; Schumann, R. Single-dose intravenous ketorolac for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2021, 5, CD013263. [Google Scholar] [CrossRef] [PubMed]
- Blank, J.J.; Berger, N.G.; Dux, J.P.; Ali, F.; Ludwig, K.A.; Peterson, C.Y. The impact of intravenous acetaminophen on pain after abdominal surgery: A meta-analysis. J. Surg. Res. 2018, 227, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, C.; Lan, L.; Liu, J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J. Clin. Anesth. 2021, 68, 110071. [Google Scholar] [CrossRef] [PubMed]
- Chincholkar, M. Gabapentinoids: Pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br. J. Pain. 2020, 14, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhu, J.; Dong, Z.; Wang, C.; Xiao, J.; Yang, W. Incidence and risk factors of postoperative nausea and vomiting following laparoscopic sleeve gastrectomy and its relationship with Helicobacter pylori: A propensity score matching analysis. Front. Endocrinol. 2023, 14, 1102017. [Google Scholar] [CrossRef]
- Kaye, A.D.; Urman, R.D.; Rappaport, Y.; Siddaiah, H.; Cornett, E.M.; Belani, K.; Salinas, O.J.; Fox, C.J. Multimodal analgesia as an essential part of enhanced recovery protocols in the ambulatory settings. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, S40–S45. [Google Scholar] [CrossRef]
- Erstad, B.L.; Barletta, J.F. Drug dosing in the critically ill obese patient-a focus on sedation, analgesia, and delirium. Crit. Care 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carron, M.; Tamburini, E.; Linassi, F.; Pettenuzzo, T.; Boscolo, A.; Navalesi, P. Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 2100. https://doi.org/10.3390/jcm13072100
Carron M, Tamburini E, Linassi F, Pettenuzzo T, Boscolo A, Navalesi P. Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(7):2100. https://doi.org/10.3390/jcm13072100
Chicago/Turabian StyleCarron, Michele, Enrico Tamburini, Federico Linassi, Tommaso Pettenuzzo, Annalisa Boscolo, and Paolo Navalesi. 2024. "Non-Opioid Analgesics and Adjuvants after Surgery in Adults with Obesity: Systematic Review with Network Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 7: 2100. https://doi.org/10.3390/jcm13072100