Efficacy and Safety of Rho Kinase Inhibitors vs. Beta-Blockers in Primary Open-Angle Glaucoma: A Systematic Review with Meta-Analysis
Abstract
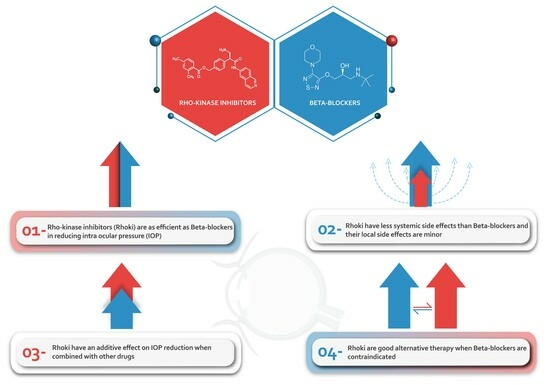
:1. Introduction
2. Materials and Methods
- Patients: performed on adults, 18 years old or more with a primary open-angle glaucoma diagnosis based on gonioscopy, OCT, and visual field defects with or without ocular hypertension.
- Intervention: Rho kinase inhibitors treatment with no additional concomitant therapy for at least 3 months.
- Comparison: beta-blockers treatment with no additional concomitant therapy for at least 3 months.
- Outcome: the reduction of IOP after a 3-month treatment was assessed by subtracting the IOP recorded at 3 months after medication from the IOP at baseline (ΔIOP = IOP at 3 months − IOP at baseline). A negative value of ΔIOP implies that the study drug is effective in lowering IOP. From each study, the mean difference of the IOP reduction was extracted at various times because IOP values can fluctuate through the day. The occurrences of systemic and topical adverse effects in both groups were also assessed.
- Case reports, systematic reviews, books, editorials, opinions, grey literature.
- Studies on types of glaucoma other than primary open-angle glaucoma.
- Studies in which additional therapies or surgical procedures for glaucoma management were also performed.
- Studies which did not specify the required data (IOP difference before and after treatment).
3. Results
3.1. Study Selection
3.2. Characteristics of Articles
3.2.1. Description of the Articles
3.2.2. Demographic Characteristics
3.2.3. Intraocular Pressure
3.2.4. Risk of Bias
3.3. Assessment of Efficacy
3.3.1. Comparison of IOP Reduction at 8 a.m.
3.3.2. Comparison of IOP Reduction at 10 a.m.
3.3.3. Comparison of IOP Reduction at 4 p.m.
3.4. Assessment of Safety
3.4.1. Topical Adverse Effects
3.4.2. Systemic Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Authors Year | Sample Size (n) | Inclusion Criteria | Follow Up. Duration | |
|---|---|---|---|---|---|
| Age (Years) Mean ± SD | Diagnosis | ||||
| ROCKET 1 | SERLE et al., 2018 [24] | 411 | 65.1 ± 11.5 | OAG or OHT >17 and <27 mmHg | Week 2 and 6, Month 3 |
| Netarsudil q.d | 202 | 65.2 ± 11.3 | |||
| Timolol b.i.d | 209 | NA | |||
| ROCKET 2 | KAHOOK et al. 2019 [25] | 756 | 65.3 ± 11.48 | OAG or OHT IOP < 25 mmHg | Week 2 and 6 Month 3, 6, 9 and 12 |
| Netarsudil q.d | 251 | NA | |||
| Timolol b.i.d | 251 | 63 ± 11.81 | |||
| Netarsudil b.i.d * | 254 | 64.1 ± 12.46 | OAG or OHT IOP < 25 mmHg | Week 2 and 6 Month 3, 6, 9 and 12 | |
| ROCKET 4 | KHOURI et al. 2019 [26] | 708 | NA | OAG or OHT IOP > 20 and <30 mmHg | Week 2 and 6, Month 3 and 6 |
| Netarsudil q.d | 351 | 64.1 ± 11.6 | |||
| Timolol b.i.d | 357 | 64.5 ± 11.0 | |||
| 8 a.m. | 10 a.m. | 4 p.m. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Netarsudil Group | Timolol Group | Netarsudil Group | Timolol Group | Netarsudil Group | Timolol Group | |||||||
| Study | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| ROCKET 1 | −3.61 | 0.62 | −4.9 | 0.51 | −3.36 | 0.62 | −3.96 | 0.55 | −3.3 | 0.62 | –3.71 | 0.51 |
| ROCKET 2 | −4.3 | 0.55 | −5.07 | 0.42 | −4.26 | 0.61 | −4.35 | 0.52 | −3.3 | 0.63 | −3.76 | 0.61 |
| ROCKET 4 | −4.52 | 41 | −5.17 | 0.55 | −4.1 | 0.63 | −4.56 | 0.51 | −3.88 | 0.55 | −3.89 | 0.61 |

References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Gallo Afflitto, G.; Aiello, F.; Cesareo, M.; Nucci, C. Primary Open Angle Glaucoma Prevalence in Europe: A Systematic Review and Meta-Analysis. J. Glaucoma 2022, 31, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Diamond, J.P. Systemic adverse effects of topical ophthalmic agents. Implications for older patients. Drugs Aging 1997, 11, 352–360. [Google Scholar] [CrossRef]
- Filimonova, E.E.; Sorokin, E.L.; Kogan, M.P.; Pashentsev, Y.E. Development of additive systemic effects when taking β-blockers in patients with glaucoma and concurrent chronic diseases. Vestn. Oftalmol. 2020, 136, 155–164. [Google Scholar] [CrossRef]
- Arai, R.; Fukamachi, D.; Monden, M.; Akutsu, N.; Murata, N.; Okumura, Y. Bradycardia Shock Caused by the Combined Use of Carteolol Eye Drops and Verapamil in an Elderly Patient with Atrial Fibrillation and Chronic Kidney Disease. Intern. Med. 2021, 60, 79–83. [Google Scholar] [CrossRef]
- Clement Freiberg, J.; von Spreckelsen, A.; Kolko, M.; Azuara-Blanco, A.; Virgili, G. Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. 2022, 6, CD013817. [Google Scholar] [PubMed]
- Abbhi, V.; Piplani, P. Rho-kinase (ROCK) Inhibitors—A Neuroprotective Therapeutic Paradigm with a Focus on Ocular Utility. Curr. Med. Chem. 2020, 27, 2222–2256. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Chang, S.-N.; Nishida, T.; Kuo, B.-I.; Lin, J.-W. Intraocular pressure-lowering efficacy and ocular safety of Rho-kinase inhibitor in glaucoma: A meta-analysis and systematic review of prospective randomized trials. Graefe Arch. Clin. Exp. Ophthalmol. 2022, 260, 937–948. [Google Scholar] [CrossRef]
- Sturdivant, J.M.; Royalty, S.M.; Lin, C.-W.; Moore, L.A.; Yingling, J.D.; Laethem, C.L.; Sherman, B.; Heintzelman, G.R.; Kopczynski, C.C.; deLong, M.A. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorg. Med. Chem. Lett. 2016, 26, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Erb, C.; Konieczka, K. Rho kinase inhibitors as new local therapy option in primary open angle glaucoma. Ophthalmologe 2021, 118, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.; Kapur, M.; Gupta, V.; Sethi, H.; Srivastava, K. Ripasudil Endgame: Role of Rho-Kinase Inhibitor as a Last-Ditch-Stand Towards Maximally Tolerated Medical Therapy to a Patient of Advanced Glaucoma. Clin. Ophthalmol. 2021, 15, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Dreischulte, T.; Lipworth, B.J.; Donnan, P.T.; Jackson, C.; Guthrie, B. Respiratory effect of beta-blocker eye drops in asthma: Population-based study and meta-analysis of clinical trials. Br. J. Clin. Pharmacol. 2016, 82, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Jolobe, O.M.P. Cardiac and extracardiac side effects of eye drops. Am. J. Emerg. Med. 2021, 46, 731. [Google Scholar] [CrossRef]
- Rains, J.; Kesterson, J. Ocular timolol as the causative agent for symptomatic bradycardia in an 89-year-old female. Am. J. Emerg. Med. 2021, 42, 263.e5–263.e6. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Serle, J.B.; Katz, L.J.; McLaurin, E.; Heah, T.; Ramirez-Davis, N.; Usner, D.W.; Novack, G.D.; Kopczynski, C.C.; ROCKET-1 and ROCKET-2 Study Groups. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients with Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 2018, 186, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Kahook, M.Y.; Serle, J.B.; Mah, F.S.; Kim, T.; Raizman, M.B.; Heah, T.; Ramirez-Davis, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D.; et al. Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am. J. Ophthalmol. 2019, 200, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Khouri, A.S.; Serle, J.B.; Bacharach, J.; Usner, D.W.; Lewis, R.A.; Braswell, P.; Kopczynski, C.C.; Heah, T.; Rocket-4 Study Group. Once-Daily Netarsudil Versus Twice-Daily Timolol in Patients with Elevated Intraocular Pressure: The Randomized Phase 3 ROCKET-4 Study. Am. J. Ophthalmol. 2019, 204, 97–104. [Google Scholar] [CrossRef]
- Testa, V.; Ferro Desideri, L.; Della Giustina, P.; Traverso, C.E.; Iester, M. An update on ripasudil for the treatment of glaucoma and ocular hypertension. Drugs Today Barc. Spain 1998 2020, 56, 599–608. [Google Scholar]
- Ottobelli, L.; Fogagnolo, P.; Frezzotti, P.; De Cillà, S.; Vallenzasca, E.; Digiuni, M.; Paderni, R.; Motolese, I.; Bagaglia, S.A.; Motolese, E.; et al. Repeatability and reproducibility of applanation resonance tonometry: A cross-sectional study. BMC Ophthalmol. 2015, 15, 36. [Google Scholar] [CrossRef]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105, 138–139. [Google Scholar]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined with Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef]
- Lee, J.-W.; Ahn, H.-S.; Chang, J.; Kang, H.-Y.; Chang, D.-J.; Suh, J.K.; Lee, H. Comparison of Netarsudil/Latanoprost Therapy with Latanoprost Monotherapy for Lowering Intraocular Pressure: A Systematic Review and Meta-analysis. Korean J. Ophthalmol. KJO 2022, 36, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Netarsudil Ophthalmic Solution 0.02%: First Global Approval. Drugs 2018, 78, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, R.; Webers, C.A.B.; Schouten, J.S.A.G.; Zeegers, M.P.; Hendrikse, F.; Prins, M.H. Intraocular pressure-lowering effects of all commonly used glaucoma drugs: A meta-analysis of randomized clinical trials. Ophthalmology 2005, 112, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.-W.; Pattabiraman, P.P.; Rao, P.V.; deLong, M.A.; Kopczynski, C.C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef]
- Ong, L.B.; Liza-Sharmini, A.T.; Chieng, L.L.; Cheong, M.T.; Vengadasalam, S.R.; Shin, H.C.; Balaravi, P. The Efficacy of Timolol in Gel-Forming Solution After Morning or Evening Dosing in Asian Glaucomatous Patients. J. Ocul. Pharmacol. Ther. 2005, 21, 388–394. [Google Scholar] [CrossRef]






| Study | Randomization Process | Deviations from Interventions | Missing Data | Outcome Measurement | Reported Results Selection | Overall Bias |
|---|---|---|---|---|---|---|
| ROCKET 1 | Low | Low | Low | Low | Low | Low |
| ROCKET 2 | Low | Low | High | Low | Low | Low |
| ROCKET 4 | Low | Low | Low | Low | Low | Low |
| Local Adverse Effects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conjunctival Hyperemia | Cornea Verticillata | Subconjunctival Hemorrhage | Blurred Vision | |||||||||
| Netarsudil | Timolol | Netarsudil | Timolol | Netarsudil | Timolol | Netarsudil | Timolol | |||||
| Study | n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p |
| ROCKET 1 | 105 (51.7) | 17 (8.1) | <0.001 | 11 (5.4) | NA | 27 (13.3) | 1 (0.5) | <0.001 | NA | NA | ||
| ROCKET 2 | 152 (60.5) | 35 (13.9) | <0.001 | 64 (25.5) | 2 (0.8) | <0.001 | 49 (19.5) | 2 (0.8) | <0.001 | 27 (10.7) | 7 (2.8) | <0.001 |
| ROCKET 4 | 168 (47.9) | 33 (9.2) | <0.001 | 86 (24.5) | 0 (0) | <0.001 | 56 (16.0) | 11 (3.1) | <0.001 | 22 (6.3) | 4 (1.1) | 0.05 |
| Systemic Adverse Effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Rate (beat/min) | Respiratory/Thoracic | Musculoskeletal | Gastrointestinal | ||||||||||
| Netarsudil | Timolol | Netarsudil | Timolol | Netarsudil | Timolol | Netarsudil | Timolol | ||||||
| Study | MD ± SD | p | MD ± SD | p | n (%) | n (%) | p | n (%) | n (%) | p | n (%) | n (%) | p |
| ROCKET 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| ROCKET 2 | NA | NS | −2.1 ± 0.6 | p < 0.001 | 10 (3.9) | 14 (5.6) | 0.5 | 3 (1.2) | 17 (6.8) | 0.01 | 2 (0.8) | 9 (3.6) | 0.06 |
| ROCKET 4 | 0.8 ± 1.0 | NS | −2 ± 1.0 | p < 0.001 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana Wandji, B.; Bacq, N.; Ehongo, A. Efficacy and Safety of Rho Kinase Inhibitors vs. Beta-Blockers in Primary Open-Angle Glaucoma: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 1747. https://doi.org/10.3390/jcm13061747
Nana Wandji B, Bacq N, Ehongo A. Efficacy and Safety of Rho Kinase Inhibitors vs. Beta-Blockers in Primary Open-Angle Glaucoma: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2024; 13(6):1747. https://doi.org/10.3390/jcm13061747
Chicago/Turabian StyleNana Wandji, Brenda, Noélie Bacq, and Adèle Ehongo. 2024. "Efficacy and Safety of Rho Kinase Inhibitors vs. Beta-Blockers in Primary Open-Angle Glaucoma: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 13, no. 6: 1747. https://doi.org/10.3390/jcm13061747