Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms
Abstract
:1. Introduction
2. Materials and Methods—See Supplemental Methods Section for Details
3. Results—See Supplemental Data and Statistics Summary Section for Details
3.1. EMMPRIN Levels in MFS TAA Tissue and Plasma
3.2. MT1-MMP Is Elevated in MFS TAA Tissue
3.3. Gelatinase Abundance in MFS TAA Tissue and Plasma
3.4. Stromelysin and Collagenase Levels in MFS TAA Tissue and Plasma
3.5. TIMP Levels in MFS TAA Tissue and Plasma
3.6. Receiver Operator Characteristic (ROC) Curve Analysis of Plasma Analytes
3.7. Effects of MT1-MMP Abundance and Localization on Secreted EMMPRIN Levels In Vitro
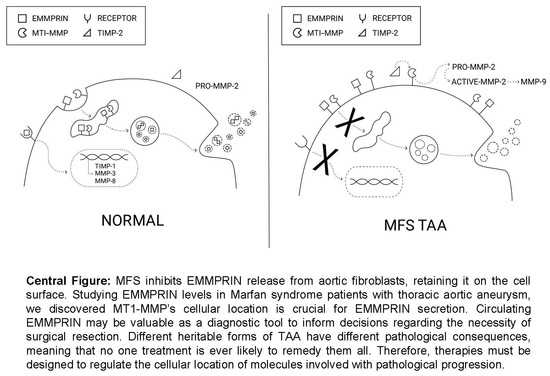
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.A.; Beck, C.; Barbour, J.R.; Zavadzkas, J.A.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: Myofibroblast-mediated vascular remodeling. Am. J. Pathol. 2009, 175, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Mann, D.L.; Entman, M.L.; Spinale, F.G. Extracellular matrix remodeling following myocardial injury. Ann. Med. 2003, 35, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Mengistab, A.T.; Tauscher, A.N.; LaVail, J.; Basbaum, C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 2004, 23, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Koshikawa, N.; Tomari, T.; Nabeshima, K.; Isobe, T.; Seiki, M. Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J. Biol. Chem. 2006, 281, 37576–37585. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, J.S.; Nadeau, E.K.; Akerman, A.W.; Stroud, R.E.; Mukherjee, R.; Jones, J.A. Regulation of membrane type-1 matrix metalloproteinase activity and intracellular localization in clinical thoracic aortic aneurysms. J. Thorac. Cardiovasc. Surg. 2017, 153, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Ruddy, J.M.; Bouges, S.; Zavadzkas, J.A.; Brinsa, T.A.; Stroud, R.E.; Mukherjee, R.; Spinale, F.G.; Ikonomidis, J.S. Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H114–H124. [Google Scholar] [CrossRef] [PubMed]
- Perrucci, G.L.; Rurali, E.; Corliano, M.; Balzo, M.; Piccoli, M.; Moschetta, D.; Pini, A.; Gaetano, R.; Antona, C.; Egea, G.; et al. Cyclophilin A/EMMPRIN Axis Is Involved in Pro-Fibrotic Processes Associated with Thoracic Aortic Aneurysm of Marfan Syndrome Patients. Cells 2020, 9, 154. [Google Scholar] [CrossRef]
- Rurali, E.; Perrucci, G.L.; Gaetano, R.; Pini, A.; Moschetta, D.; Gentilini, D.; Nigro, P.; Pompilio, G. Soluble EMMPRIN levels discriminate aortic ectasia in Marfan syndrome patients. Theranostics 2019, 9, 2224–2234. [Google Scholar] [CrossRef]
- Ni, K.; Wang, C.; Carnino, J.M.; Jin, Y. The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles. Med. Sci. 2020, 8, 46. [Google Scholar] [CrossRef]
- Lin, W.; Xiong, J.; Jiang, Y.; Liu, H.; Bian, J.; Wang, J.; Shao, Y.; Ni, B. Fibrillin-1 mutation contributes to Marfan syndrome by inhibiting Cav1.2-mediated cell proliferation in vascular smooth muscle cells. Channels 2023, 17, 2192377. [Google Scholar] [CrossRef]
- Diehl, J.N.; Ray, A.; Collins, L.B.; Peterson, A.; Alexander, K.C.; Boutros, J.G.; Ikonomidis, J.S.; Akerman, A.W. A standardized method for plasma extracellular vesicle isolation and size distribution analysis. PLoS ONE 2023, 18, e0284875. [Google Scholar] [CrossRef]
- Akerman, A.W.; Collins, E.N.; Peterson, A.R.; Collins, L.B.; Harrison, J.K.; DeVaughn, A.; Townsend, J.M.; Vanbuskirk, R.L.; Riopedre-Maqueira, J.; Reyes, A.; et al. miR-133a Replacement Attenuates Thoracic Aortic Aneurysm in Mice. J. Am. Heart Assoc. 2021, 10, e019862. [Google Scholar] [CrossRef]
- Itoh, Y.; Takamura, A.; Ito, N.; Maru, Y.; Sato, H.; Suenaga, N.; Aoki, T.; Seiki, M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001, 20, 4782–4793. [Google Scholar] [CrossRef] [PubMed]
- Chellaiah, M.A.; Ma, T. Membrane localization of membrane type 1 matrix metalloproteinase by CD44 regulates the activation of pro-matrix metalloproteinase 9 in osteoclasts. Biomed. Res. Int. 2013, 2013, 302392. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998, 8, 179–186. [Google Scholar] [CrossRef]
- Kataoka, H.; DeCastro, R.; Zucker, S.; Biswas, C. Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 1993, 53, 3154–3158. [Google Scholar] [PubMed]
- Yanaba, K.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Hamaguchi, Y.; Sato, S. Increased serum soluble CD147 levels in patients with systemic sclerosis: Association with scleroderma renal crisis. Clin. Rheumatol. 2012, 31, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.D.A.; Jadli, A.S.; Fedak, P.W.M.; Patel, V.B. Adventitial Fibroblasts in Aortic Aneurysm: Unraveling Pathogenic Contributions to Vascular Disease. Diagnostics 2022, 12, 871. [Google Scholar] [CrossRef] [PubMed]
- Akkus, M.N.; Ormam, A.; Seyis, S.; Baran, C.; Gorur, A.; Bilen, M.N. Plasma EMMPRIN levels in acute myocardial infarction and stable coronary artery disease. Clin. Invest. Med. 2016, 39, E79–E87. [Google Scholar] [CrossRef] [PubMed]
- Knutti, N.; Kuepper, M.; Friedrich, K. Soluble extracellular matrix metalloproteinase inducer (EMMPRIN, EMN) regulates cancer-related cellular functions by homotypic interactions with surface CD147. FEBS J. 2015, 282, 4187–4200. [Google Scholar] [CrossRef]
- Lee, A.; Rode, A.; Nicoll, A.; Maczurek, A.E.; Lim, L.; Lim, S.; Angus, P.; Kronborg, I.; Arachchi, N.; Gorelik, A.; et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hao, Z.W.; Zhao, Y.X.; Yang, X.M.; Tang, H.; Zhang, X.; Song, F.; Sun, X.X.; Wang, B.; Nan, G.; et al. Full-length soluble CD147 promotes MMP-2 expression and is a potential serological marker in detection of hepatocellular carcinoma. J. Transl. Med. 2014, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.K.; Yong, H.Y.; Hahn, J.N.; Silva, C.; Casha, S.; Hurlbert, R.J.; Jacques, F.H.; Lisak, R.; Khan, O.; Ionete, C.; et al. Evaluating Soluble EMMPRIN as a Marker of Disease Activity in Multiple Sclerosis: Studies of Serum and Cerebrospinal Fluid. PLoS ONE 2016, 11, e0163802. [Google Scholar] [CrossRef]
- Rozado, J.; Martin, M.; Pascual, I.; Hernandez-Vaquero, D.; Moris, C. Comparing American, European and Asian practice guidelines for aortic diseases. J. Thorac. Dis. 2017, 9, S551–S560. [Google Scholar] [CrossRef]
- Elefteriades, J.A.; Farkas, E.A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J. Am. Coll. Cardiol. 2010, 55, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar] [PubMed]
- Collen, A.; Hanemaaijer, R.; Lupu, F.; Quax, P.H.; van Lent, N.; Grimbergen, J.; Peters, E.; Koolwijk, P.; van Hinsbergh, V.W. Membrane-type matrix metalloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood 2003, 101, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F., Jr. MMPs and TIMPs. An historical perspective. Methods Mol. Biol. 2001, 151, 1–23. [Google Scholar]
- Stawowy, P.; Meyborg, H.; Stibenz, D.; Borges Pereira Stawowy, N.; Roser, M.; Thanabalasingam, U.; Veinot, J.P.; Chretien, M.; Seidah, N.G.; Fleck, E.; et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation 2005, 111, 2820–2827. [Google Scholar] [CrossRef]
- Manso, A.M.; Elsherif, L.; Kang, S.M.; Ross, R.S. Integrins, membrane-type matrix metalloproteinases and ADAMs: Potential implications for cardiac remodeling. Cardiovasc. Res. 2006, 69, 574–584. [Google Scholar] [CrossRef]
- Strongin, A.Y.; Collier, I.; Bannikov, G.; Marmer, B.L.; Grant, G.A.; Goldberg, G.I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J. Biol. Chem. 1995, 270, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Juttermann, R.; Soloway, P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000, 275, 26411–26415. [Google Scholar] [CrossRef] [PubMed]
- Galvez, B.G.; Matias-Roman, S.; Yanez-Mo, M.; Vicente-Manzanares, M.; Sanchez-Madrid, F.; Arroyo, A.G. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol. Biol. Cell 2004, 15, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Hemler, M.E. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J. Biol. Chem. 2004, 279, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Teeters, K.; Miyasato, S.; Choi, J.; Nakamatsu, G.; Richardson, J.A.; Starcher, B.; Davis, E.C.; Tam, E.K.; Jourdan-Le Saux, C. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L1007–L1017. [Google Scholar] [CrossRef]
- Sabatier, L.; Chen, D.; Fagotto-Kaufmann, C.; Hubmacher, D.; McKee, M.D.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Fibrillin assembly requires fibronectin. Mol. Biol. Cell 2009, 20, 846–858. [Google Scholar] [CrossRef]
- Bonnema, D.D.; Webb, C.S.; Pennington, W.R.; Stroud, R.E.; Leonardi, A.E.; Clark, L.L.; McClure, C.D.; Finklea, L.; Spinale, F.G.; Zile, M.R. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J. Card. Fail. 2007, 13, 530–540. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexander, K.C.; Anderson, C.W.; Agala, C.B.; Tasoudis, P.; Collins, E.N.; Ding, Y.; Blackwell, J.W.; Willcox, D.E.; Farivar, B.S.; Kibbe, M.R.; et al. Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms. J. Clin. Med. 2024, 13, 1548. https://doi.org/10.3390/jcm13061548
Alexander KC, Anderson CW, Agala CB, Tasoudis P, Collins EN, Ding Y, Blackwell JW, Willcox DE, Farivar BS, Kibbe MR, et al. Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms. Journal of Clinical Medicine. 2024; 13(6):1548. https://doi.org/10.3390/jcm13061548
Chicago/Turabian StyleAlexander, Kyle C., Carlton W. Anderson, Chris B. Agala, Panagiotis Tasoudis, Elizabeth N. Collins, Yiwen Ding, John W. Blackwell, Danielle E. Willcox, Behzad S. Farivar, Melina R. Kibbe, and et al. 2024. "Paradoxical Changes: EMMPRIN Tissue and Plasma Levels in Marfan Syndrome-Related Thoracic Aortic Aneurysms" Journal of Clinical Medicine 13, no. 6: 1548. https://doi.org/10.3390/jcm13061548