Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate/High-Risk Pulmonary Embolism: Design of the Multicenter USAT IH-PE Registry and Preliminary Results
Abstract
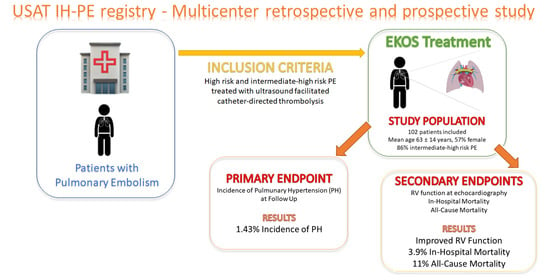
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Statistical Analysis
3. Results
3.1. Outcomes
3.1.1. The Primary Endpoint
3.1.2. Secondary Efficacy Endpoint
3.1.3. Mortality
3.1.4. Secondary Safety Endpoint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 13401347. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.P.; Munzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef]
- Harvey, J.J.; Huang, S.; Uberoi, R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst. Rev. 2022, 2022, CD013083. [Google Scholar]
- Braaten, J.V.; A Goss, R.; Francis, C.W. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997, 78, 1063–1068. [Google Scholar] [CrossRef]
- Francis, C.W.; Blinc, A.; Lee, S.; Cox, C. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med. Biol. 1995, 21, 419–424. [Google Scholar] [CrossRef]
- Siddiqi, F.; Odrljin, T.M.; Fay, P.J.; Cox, C.; Francis, C.W. Binding of tissue-plasminogen activator to fibrin: Effect of ultrasound. Blood 1998, 91, 2019–2025. [Google Scholar] [CrossRef]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; Jones, N.J. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef]
- Kuo, W.T.; Banerjee, A.; Kim, P.S.; DeMarco, F.J.; Levy, J.R.; Facchini, F.R.; De Gregorio, M.A. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results from a Prospective Multicenter Registry. Chest 2015, 148, 667–673. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; Baumgartner, I. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; Goldhaber, S.Z. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Seeger, W.; Sommer, N.; Wilhelm, J.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Pinsky, M.R.; Hc, D.; Naeije, R.; Deible, C.; Kosaraju, V.; Simon, M.A. Right Ventricular-Pulmonary Arterial Coupling Predicts Outcome in Patients Referred for Pulmonary Hypertension. Heart 2015, 101, 37–43. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Tua, L.; Mandurino-Mirizzi, A.; Colombo, C.; Morici, N.; Magrini, G.; Nava, S.; Frassica, R.; Montalto, C.; Ferlini, M.; Sacco, A.; et al. The impact of transcatheter edge-to-edge repair on right ventricle-pulmonary artery coupling in patients with functional mitral regurgitation. Eur. J. Clin. Investig. 2023, 53, e13869. [Google Scholar] [CrossRef]
- Lyhne, M.D.; Kabrhel, C.; Giordano, N.; Andersen, A.; Nielsen-Kudsk, J.E.; Zheng, H.; Dudzinski, D.M. The echocardiographic ratio tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure predicts short-term adverse outcomes in acute pulmonary embolism. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 285–294. [Google Scholar] [CrossRef]
- Forton, K.; Motoji, Y.; Caravita, S.; Faoro, V.; Naeije, R. Exercise stress echocardiography of the pulmonary circulation and right ventricular-arterial coupling in healthy adolescents. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 688–694. [Google Scholar] [CrossRef]
- Wolsk, E.; Bakkestrøm, R.; Kristensen, C.B.; Myhr, K.A.; Thomsen, J.H.; Balling, L.; Andersen, M.J.; Dahl, J.S.; Shah, S.J.; Gustafsson, F.; et al. Right Ventricular and Pulmonary Vascular Function are Influenced by Age and Volume Expansion in Healthy Humans. J. Card Fail. 2019, 25, 51–59. [Google Scholar] [CrossRef]
- Ferrara, F.; Rudski, L.G.; Vriz, O.; Gargani, L.; Afilalo, J.; D’Andrea, A.; D'Alto, M.; Marra, A.M.; Acri, E.; Stanziola, A.A.; et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int. J. Cardiol. 2016, 223, 736–743. [Google Scholar] [CrossRef]
- Pietrasik, A.; Gąsecka, A.; Szarpak, Ł.; Pruc, M.; Kopiec, T.; Darocha, S.; Banaszkiewicz, M.; Niewada, M.; Grabowski, M.; Kurzyna, M. Catheter-Based Therapies Decrease Mortality in Patients With Intermediate and High-Risk Pulmonary Embolism: Evidence From Meta-Analysis of 65,589 Patients. Front. Cardiovasc. Med. 2022, 9, 861307. [Google Scholar] [CrossRef]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-directed therapy for the treatment of massive pulmonary embolism: Systematic review and meta-analysis of modern techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef]
- Poenou, G.; Dumitru, T.D.; Lafaie, L.; Mismetti, V.; Ayoub, E.; Duvillard, C.; Accassat, S.; Mismetti, P.; Heestermans, M.; Bertoletti, L. Pulmonary Embolism in the Cancer Associated Thrombosis Landscape. J. Clin. Med. 2022, 11, 5650. [Google Scholar] [CrossRef]
- Weeda, E.R.; Hakamiun, K.M.; Leschorn, H.X.; Tran, E. Comorbid cancer and use of thrombolysis in acute pulmonary embolism. J. Thromb. Thrombolysis 2019, 47, 324–327. [Google Scholar] [CrossRef]
- Shalaby, K.; Kahn, A.; Silver, E.S.; Kim, M.J.; Balakumaran, K.; Kim, A.S. Outcomes of acute pulmonary embolism in hospitalized patients with cancer. BMC Pulm. Med. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Piazza, G. Multidisciplinary PE response teams. Circulation 2016, 133, 98103. [Google Scholar] [CrossRef] [PubMed]


| Baseline Demographics | |
|---|---|
| Female | 58 (56.9) |
| Age | 63.7 ± 14.5 |
| Smoking current past | 8 (7.8) 15 (14.7) |
| BMI | 28.1 ± 5.2 |
| Hypertension | 58 (56.9) |
| Cancer | 29 (28.4) |
| Previous DVP | 21 (20.6) |
| Heart failure | 6 (5.9) |
| CAD | 6 (5.9) |
| Previous stroke | 8 (7.8) |
| Previous PE | 9 (8.8) |
| Previous DVP | 21 (20.6) |
| Diabetes mellitus | 13 (12.7) |
| Chronic kidney disease | 11 (10.8) |
| Presenting symptoms | |
| Dyspnea | 75 (73.5) |
| Chest pain | 23 (22.5) |
| Syncope | 20 (19.6) |
| Hemoptysis | 0 |
| Peripheral edema | 8 (7.8) |
| Heart palpitations | 4 (3.9) |
| Clinical presentation | |
| Systolic blood pressure, mm Hg | 127 ± 24 |
| Diastolic blood pressure, mmHg | 76 ± 15 |
| Heart rate, BPM | 103 ± 19 |
| Oxygen saturation, % | 95 ± 4 |
| First available PaO2/FiO2 | 244 ± 102 |
| Sepsis | 2 (2) |
| Neurological alteration | 8 (8.1) |
| Laboratory | |
| Hemoglobin (g/dL) | 12.9 ± 2 |
| Platelets (n × 103/mm3) | 221 ± 79 |
| WBC, ×103/μL | 12 ± 8 |
| D-dimer, ng/mL | 16.8 ± 11.1 |
| Creatinine, mg/dL | 1.1 ± 0.6 |
| Troponin on admission ng/L | 102.6 ± 111.4 |
| NT-proBNP, ng/L | 3948.7 ± 6374.8 |
| Arterial lactate, mmol/L | 2.5 ± 2 |
| Characteristics of PE at CT scan | |
| Bilateral main pulmonary arteries | 77 (75.5) |
| Unilateral main pulmonary arteries | 7 (6.9) |
| Bilateral, lobar | 15 (14.7) |
| Unilateral, lobar | 3 (3.6) |
| T 0 | T24 | T-FU | |
|---|---|---|---|
| TAPSE ≤ 16 mm TAPSE (mm) ΔTAPSE | 56 (63) 16 ± 3 | 19 (19.8) 19.5 ± 3.6 3.4 ± 2.8 | 3 (4.5) 22.5 ± 3.5 6.2 ± 3.1 |
| PASP ≥ 40 mmHg PASP (mmHg) ΔPASP | 68 (83) 48 ± 13 | 29 (34) 38 ± 11 −9.9 ± 9.7 | 4 (7) 31 ± 7 −17.4 ± 9.6 |
| RV/LV > 1 RV/LV ΔRV/LV | 27 (48) 1 ± 0.2 | 15 (18) 0.9 ± 0.2 −0.2 ± 0.2 | 0 (0) 0.8 ± 0.1 −0.2 ± 0.1 |
| TAPSE/PASP ΔTAPSE/PASP | 0.3 ± 0.1 | 0.5 ± 0.9 0.2 ± 0.1 | 0.8 ± 0.2 0.4 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, C.; Capsoni, N.; Russo, F.; Iannaccone, M.; Adamo, M.; Viola, G.; Bossi, I.E.; Villanova, L.; Tognola, C.; Curci, C.; et al. Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate/High-Risk Pulmonary Embolism: Design of the Multicenter USAT IH-PE Registry and Preliminary Results. J. Clin. Med. 2024, 13, 619. https://doi.org/10.3390/jcm13020619
Colombo C, Capsoni N, Russo F, Iannaccone M, Adamo M, Viola G, Bossi IE, Villanova L, Tognola C, Curci C, et al. Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate/High-Risk Pulmonary Embolism: Design of the Multicenter USAT IH-PE Registry and Preliminary Results. Journal of Clinical Medicine. 2024; 13(2):619. https://doi.org/10.3390/jcm13020619
Chicago/Turabian StyleColombo, Claudia, Nicolò Capsoni, Filippo Russo, Mario Iannaccone, Marianna Adamo, Giovanna Viola, Ilaria Emanuela Bossi, Luca Villanova, Chiara Tognola, Camilla Curci, and et al. 2024. "Ultrasound-Assisted, Catheter-Directed Thrombolysis for Acute Intermediate/High-Risk Pulmonary Embolism: Design of the Multicenter USAT IH-PE Registry and Preliminary Results" Journal of Clinical Medicine 13, no. 2: 619. https://doi.org/10.3390/jcm13020619