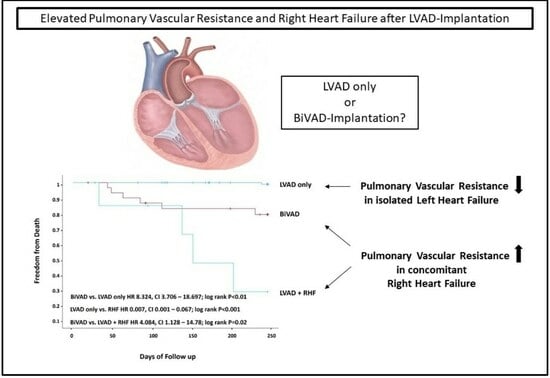
Pulmonary Vascular Resistance to Predict Right Heart Failure in Patients Undergoing Left Ventricular Assist Device Implantation
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Assessments
2.3. Right Heart Failure
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Left Ventricular Function and Invasive Hemodynamics
3.3. Outcomes
3.4. Subgroups and Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2DE | two-dimensional transthoracic echocardiography |
| CI | confidence interval |
| CT | computed tomography |
| EACVI | European Association of Cardiovascular Imaging |
| HF | heart failure |
| HR | hazard ratio |
| LVAD | left ventricular assist device |
| LVEF | left ventricular ejection fraction |
| PVR | pulmonary vascular resistance |
| RHC | right heart catheterization |
| RHF | right heart failure |
| ROC | receiver operating characteristic |
| RVAD | right ventricular assist device |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Fox, H.; Marrouche, N.F.; Crijns, H.; Costard-Jaeckle, A.; Bergau, L.; Hindricks, G.; Dagres, N.; Sossalla, S.; Schramm, R.; et al. Catheter Ablation in End-Stage Heart Failure with Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Morshuis, M.; Fox, H.; Lauenroth, V.; Schramm, R. Long-term assist device patients admitted to ICU: Tips and pitfalls. J. Intensive Med. 2023, 3, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Alkhunaizi, F.A.; Azih, N.I.; Read, J.M.; Goldberg, R.L.; Gulati, A.A.; Scheel, P.J., 3rd; Muslem, R.; Gilotra, N.A.; Sharma, K.; Kilic, A.; et al. Characteristics and Predictors of Late Right Heart Failure after Left Ventricular Assist Device Implantation. ASAIO J. 2023, 69, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.C.; Topkara, V.K.; Mercando, M.; Kay, J.; Kruger, K.H.; Aboodi, M.S.; Oz, M.C.; Naka, Y. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J. Heart Lung Transplant. 2006, 25, 1–6. [Google Scholar] [CrossRef]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef]
- Rich, J.D.; Gosev, I.; Patel, C.B.; Joseph, S.; Katz, J.N.; Eckman, P.M.; Lee, S.; Sundareswaran, K.; Kilic, A.; Bethea, B.; et al. The incidence, risk factors, and outcomes associated with late right-sided heart failure in patients supported with an axial-flow left ventricular assist device. J. Heart Lung Transplant. 2017, 36, 50–58. [Google Scholar] [CrossRef]
- Hahn, R.T.; Lerakis, S.; Delgado, V.; Addetia, K.; Burkhoff, D.; Muraru, D.; Pinney, S.; Friedberg, M.K. Multimodality Imaging of Right Heart Function: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1954–1973. [Google Scholar] [CrossRef]
- Pickett, C.A.; Cheezum, M.K.; Kassop, D.; Villines, T.C.; Hulten, E.A. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two- and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: A meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 848–852. [Google Scholar] [CrossRef]
- Rizvi, A.; Deaño, R.C.; Bachman, D.P.; Xiong, G.; Min, J.K.; Truong, Q.A. Analysis of ventricular function by CT. J. Cardiovasc. Comput. Tomogr. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Sugeng, L.; Mor-Avi, V.; Weinert, L.; Niel, J.; Ebner, C.; Steringer-Mascherbauer, R.; Bartolles, R.; Baumann, R.; Schummers, G.; Lang, R.M.; et al. Multimodality comparison of quantitative volumetric analysis of the right ventricle. JACC Cardiovasc. Imaging 2010, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Echocardiographic assessment of the right ventricle: Impact of the distinctly load dependency of its size, geometry and performance. Int. J. Cardiol. 2016, 221, 1132–1142. [Google Scholar] [CrossRef]
- Gyoten, T.; Rojas, S.V.; Fox, H.; Hata, M.; Deutsch, M.A.; Schramm, R.; Gummert, J.F.; Morshuis, M. Cardiac recovery following left ventricular assist device therapy: Experience of complete device explantation including ventricular patch plasty. Eur. J. Cardiothorac. Surg. 2021, 59, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.H. Pulmonary Hypertension by the Method of Paul Wood. Chest 2020, 158, 1164–1171. [Google Scholar] [CrossRef]
- Maron, B.A.; Brittain, E.L.; Hess, E.; Waldo, S.W.; Barón, A.E.; Huang, S.; Goldstein, R.H.; Assad, T.; Wertheim, B.M.; Alba, G.A.; et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Gual-Capllonch, F.; Teis, A.; Ferrer, E.; Núñez, J.; Vallejo, N.; Juncà, G.; López-Ayerbe, J.; Lupón, J.; Bayes-Genis, A. Pulmonary vascular resistance versus pulmonary artery pressure for predicting right ventricular remodeling and functional tricuspid regurgitation. Echocardiography 2018, 35, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Stocker, T.J.; Hertell, H.; Orban, M.; Braun, D.; Rommel, K.P.; Ruf, T.; Ong, G.; Nabauer, M.; Deseive, S.; Fam, N.; et al. Cardiopulmonary Hemodynamic Profile Predicts Mortality After Transcatheter Tricuspid Valve Repair in Chronic Heart Failure. JACC Cardiovasc. Interv. 2021, 14, 29–38. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.H.; Kim, J.; Park, S.J.; Park, M.S.; Choi, G.S.; Lee, S.K.; Kim, G.S. Predictive Value of Intraoperative Pulmonary Vascular Resistance in Liver Transplantation. Liver Transpl. 2018, 24, 1680–1689. [Google Scholar] [CrossRef]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Fitzpatrick, J.R., 3rd; Frederick, J.R.; Hsu, V.M.; Kozin, E.D.; O’Hara, M.L.; Howell, E.; Dougherty, D.; McCormick, R.C.; Laporte, C.A.; Cohen, J.E.; et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J. Heart Lung Transplant. 2008, 27, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.I.I.; Akin, S.; Muslem, R.; Boersma, E.; Manintveld, O.C.; Krabatsch, T.; Gummert, J.F.; de By, T.; Bogers, A.; Zijlstra, F.; et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018, 137, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Ancona, F.; Brochet, E.; Donal, E.; Dweck, M.; Faletra, F.; Lancellotti, P.; Mahmoud-Elsayed, H.; Marsan, N.A.; Maurovich-Hovart, P.; et al. The structural heart disease interventional imager rationale, skills and training: A position paper of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 471–479. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Farber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Cleveland, J.C., Jr.; Naftel, D.C.; Reece, T.B.; Murray, M.; Antaki, J.; Pagani, F.D.; Kirklin, J.K. Survival after biventricular assist device implantation: An analysis of the Interagency Registry for Mechanically Assisted Circulatory Support database. J. Heart Lung Transplant. 2011, 30, 862–869. [Google Scholar] [CrossRef]
- Rojas, S.V.; Junghans, S.; Fox, H.; Lazouski, K.; Schramm, R.; Morshuis, M.; Gummert, J.F.; Gross, J. Bacteriophage-Enriched Galenic for Intrapericardial Ventricular Assist Device Infection. Antibiotics 2022, 11, 602. [Google Scholar] [CrossRef]
- Mullan, C.; Caraballo, C.; Ravindra, N.G.; Miller, P.E.; Mori, M.; McCullough, M.; Clarke, J.D.; Anwer, M.; Velazquez, E.J.; Geirsson, A.; et al. Clinical impact of concomitant tricuspid valve procedures during left ventricular assist device implantation. J. Heart Lung Transplant. 2020, 39, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Schweiger, M.; Stepanenko, A.; Dandel, M.; Kukucka, M.; Vierecke, J.; Hetzer, R.; Krabatsch, T. Tricuspid valve repair in patients supported with left ventricular assist devices. Asaio J. 2011, 57, 363–367. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Total (n = 129) | LVAD Only (n = 95) | LVAD + RHF (n = 34) | p-Value |
|---|---|---|---|---|
| Age, years | 56 ± 11 | 57 ± 10 | 54 ± 13 | 0.194 |
| Male sex, n (%) | 116 (89%) | 84 (88%) | 32 (94%) | 0.512 |
| Urea, mg/dL | 70 ± 43 | 69 ± 42.6 | 71.8 ± 46 | 0.794 |
| Creatinine, mg/dL | 1.6 ± 1 | 1.5 ± 1 | 1.7 ± 1 | 0.845 |
| Bilirubin, mg/dL | 1.5 ± 1.6 | 1.4 ± 1.2 | 2 ± 2.5 | 0.196 |
| ALT, U/L | 70 ± 158 | 71 ± 179 | 65 ± 79 | 0.422 |
| AST, U/L | 51 ± 105 | 53 ± 120 | 47 ± 40 | 0.784 |
| CRP, mg/dL | 3.4 ± 4.9 | 3.0 ± 4.4 | 4.4 ± 6 | 0.154 |
| Parameter | Total (n = 129) | LVAD Only (n = 95) | LVAD + RHF (n = 34) | p-Value |
|---|---|---|---|---|
| LVEF, % | 21.5 ± 6.2 | 21.5 ± 6.2 | 21.5 ± 6.5 | 0.961 |
| LVEDD, mm | 72 ± 11 | 71 ± 11 | 73 ± 13 | 0.51 |
| LVESD, mm | 66 ± 12 | 66 ± 11 | 65 ± 15 | 0.732 |
| Heart rate, beats/min | 87 ± 18 | 86 ± 17 | 89 ± 20 | 0.414 |
| Mean PAP, mmHg | 33 ± 11 | 32 ± 7 | 33 ± 9 | 0.983 |
| PCWP, mmHg | 22 ± 10 | 22 ± 11 | 22 ± 7 | 0.94 |
| CO, L/min | 5.1 ± 7.9 | 4.7 ± 4.9 | 6.2 ± 13.3 | 0.339 |
| CI, L/min/m2 | 2.1 ± 0.64 | 2.1 ± 0.67 | 2.02 ± 0.59 | 0.515 |
| SVR, dyn/s/cm5 | 4152 ± 1483 | 1354 ± 620 | 1904 ± 942 | 0.008 |
| PVR, dyn/s/cm5 | 269 ± 231 | 234 ± 162 | 404 ± 375 | 0.01 |
| Inotropes | Total (n = 129) | LVAD Only (n = 95) | LVAD + RHF (n = 34) | p-Value |
|---|---|---|---|---|
| Milrinone | 67 (52%) | 49 (52%) | 18 (53%) | 0.99 |
| Dobutamine | 64 (50%) | 49 (52%) | 15 (44%) | 0.55 |
| Levosimendan | 2 (2%) | 2 (2%) | 0 (0%) | 0.99 |
| Epinephrine | 2 (2%) | 1 (1%) | 1 (3%) | 0.459 |
| Dopamine | 14 (11%) | 5 (5%) | 9 (26%) | 0.002 |
| Norepinephrine | 3 (2%) | 3 (3%) | 0 (0%) | 0.566 |
| Total (n = 129) | LVAD Only (n = 95) | LVAD + RHF (n = 34) | p-Value | |
|---|---|---|---|---|
| Death, n (%) | 11 (8.5) | 1 (1.1) | 10 (29.4) | <0.01 |
| Duration of invasive mechanical ventilation, days | 227 ± 535 | 101 ± 200 | 602 ± 922 | <0.001 |
| Duration of ICU stay, days | 28.4 ± 39.5 | 15.5 ± 19.7 | 64.4 ± 56 | <0.001 |
| LVEF, % | 24.6 ± 8 | 23.9 ± 6.5 | 26.8 ± 11 | 0.068 |
| LVEDD, mm | 59 ± 13 | 59 ± 13 | 58 ± 13 | 0.516 |
| LVESD, mm | 55 ± 13 | 55 ± 13 | 54 ± 14 | 0.729 |
| Urea, mg/dL | 52 ± 33 | 49 ± 31 | 61 ± 38 | 0.094 |
| Creatinine, mg/dL | 1.3 ± 1.1 | 1.3 1.7 | 1.4 ± 0.8 | 0.697 |
| Bilirubin, mg/dL | 1 ± 1.9 | 0.68 ± 0.25 | 1.9 ± 0.25 | 0.06 |
| ALT, U/L | 24 ± 26 | 19 ± 13 | 37 ± 42 | 0.045 |
| AST, U/L | 33 ± 34 | 27 ± 10 | 47 ± 63 | 0.104 |
| CRP, mg/dL | 4.1 ± 4.2 | 3.4 ± 3.1 | 5.9 ± 6.1 | 0.96 |
| Univariate Cox Regression | Multivariate Cox Regression | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.00 (0.95–1.06) | 0.916 | - | |
| Sex | 0.88 (0.11–6.90) | 0.905 | - | |
| LVEF, % | 1.09 (0.99–1.20) | 0.079 | 1.07 (0.95–1.19) | 0.272 |
| Bilirubin, mg/dL | 1.21 (1.04–1.40) | 0.012 | 0.53 (1.57–1.79) | 0.307 |
| Urea, mg/dL | 1.00 (0.98–1.01) | 0.775 | - | |
| ALT, U/L | 1.00 (0.99–1.01) | 0.914 | - | |
| AST, U/L | 0.99 (0.98–1.01) | 0.437 | - | |
| Creatinine, mg/dL | 1.20 (0.77–1.86) | 0.419 | - | |
| Mean PAP, mmHg | 1.01 (0.95–1.06) | 0.817 | - | |
| PVR >250 vs. <250 dyn/s/cm5 | 10.89 (1.31–90.50) | 0.027 | 10.38 (1.21–89.04) | 0.033 |
| SVR, dyn/s/cm5 | 1 (1.00–1.00) | 0.773 | - | |
| PCWP, mmHg | 0.99 (0.93–1.07) | 0.867 | - | |
| Cardiac index | 0.93 (0.37–2.33) | 0.872 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schramm, R.; Kirchner, J.; Ibrahim, M.; Rojas, S.V.; Morshuis, M.; Rudolph, V.; Gummert, J.F.; Fox, H. Pulmonary Vascular Resistance to Predict Right Heart Failure in Patients Undergoing Left Ventricular Assist Device Implantation. J. Clin. Med. 2024, 13, 462. https://doi.org/10.3390/jcm13020462
Schramm R, Kirchner J, Ibrahim M, Rojas SV, Morshuis M, Rudolph V, Gummert JF, Fox H. Pulmonary Vascular Resistance to Predict Right Heart Failure in Patients Undergoing Left Ventricular Assist Device Implantation. Journal of Clinical Medicine. 2024; 13(2):462. https://doi.org/10.3390/jcm13020462
Chicago/Turabian StyleSchramm, René, Johannes Kirchner, Mohamad Ibrahim, Sebastian V. Rojas, Michiel Morshuis, Volker Rudolph, Jan F. Gummert, and Henrik Fox. 2024. "Pulmonary Vascular Resistance to Predict Right Heart Failure in Patients Undergoing Left Ventricular Assist Device Implantation" Journal of Clinical Medicine 13, no. 2: 462. https://doi.org/10.3390/jcm13020462