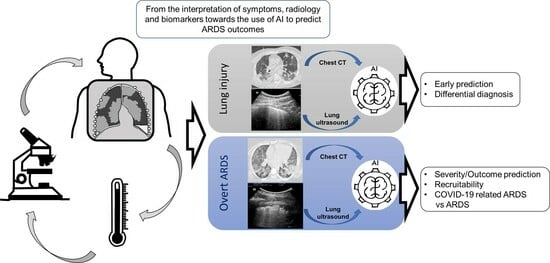
Lung Imaging and Artificial Intelligence in ARDS
Abstract
:1. Introduction
2. Machine Learning and Deep Learning
3. Search Strategy
4. Computed Tomography Scan
4.1. Prediction of ARDS
4.2. Alveolar Recruitment
4.3. Outcome
5. Lung Ultrasound
5.1. Prediction of ARDS Diagnosis
5.2. Differential Diagnosis
5.3. Limitations of AI in LUS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haug, C.J.; Drazen, J.M. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Pennati, F.; Aliverti, A.; Pozzi, T.; Gattarello, S.; Lombardo, F.; Coppola, S.; Chiumello, D. Machine Learning Predicts Lung Recruitment in Acute Respiratory Distress Syndrome Using Single Lung CT Scan. Ann. Intensive Care 2023, 13, 60. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Biswas, M.; Saba, L.; Bit, A.; Tandel, G.S.; Agarwal, M.; Patrick, A.; et al. A Narrative Review on Characterization of Acute Respiratory Distress Syndrome in COVID-19-Infected Lungs Using Artificial Intelligence. Comput. Biol. Med. 2021, 130, 104210. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.; Agarwal, S.; Coombs, L.; Wald, C.; Dreyer, K. 2020 ACR Data Science Institute Artificial Intelligence Survey. J. Am. Coll. Radiol. 2021, 18, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Papa, G.F.S.; Artigas, A.; Bouhemad, B.; Grgic, A.; Heunks, L.; Markstaller, K.; Pellegrino, G.M.; Pisani, L.; Rigau, D.; et al. ERS Statement on Chest Imaging in Acute Respiratory Failure. Eur. Respir. J. 2019, 54, 1900435. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Badiei Khuzani, M.; Vasudevan, V.; Huang, C.; Ren, H.; Xiao, R.; Jia, X.; Xing, L. Machine Learning Techniques for Biomedical Image Segmentation: An Overview of Technical Aspects and Introduction to State-of-Art Applications. Med. Phys. 2020, 47, e148–e167. [Google Scholar] [CrossRef]
- Hinton, G.E.; Osindero, S.; Teh, Y.W. A Fast Learning Algorithm for Deep Belief Nets. Neural Comput. 2006, 18, 1527–1554. [Google Scholar] [CrossRef]
- Nishiyama, A.; Kawata, N.; Yokota, H.; Sugiura, T.; Matsumura, Y.; Higashide, T.; Horikoshi, T.; Oda, S.; Tatsumi, K.; Uno, T. A Predictive Factor for Patients with Acute Respiratory Distress Syndrome: CT Lung Volumetry of the Well-Aerated Region as an Automated Method. Eur. J. Radiol. 2020, 122, 108748. [Google Scholar] [CrossRef]
- Gresser, E.; Reich, J.; Sabel, B.O.; Kunz, W.G.; Fabritius, M.P.; Rübenthaler, J.; Ingrisch, M.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; et al. Risk Stratification for Ecmo Requirement in Covid-19 Icu Patients Using Quantitative Imaging Features in Ct Scans on Admission. Diagnostics 2021, 11, 1029. [Google Scholar] [CrossRef]
- Herrmann, P.; Busana, M.; Cressoni, M.; Lotz, J.; Moerer, O.; Saager, L.; Meissner, K.; Quintel, M.; Gattinoni, L. Using Artificial Intelligence for Automatic Segmentation of CT Lung Images in Acute Respiratory Distress Syndrome. Front. Physiol. 2021, 12, 676118. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Hong, K.S.; Chikontwe, P.; Luna, M.; Jang, J.G.; Park, J.; Shin, K.C.; Park, S.H.; Ahn, J.H. Quantitative Assessment of Chest CT Patterns in COVID-19 and Bacterial Pneumonia Patients: A Deep Learning Perspective. J. Korean Med. Sci. 2021, 36, e46. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative Chest CT Analysis in COVID-19 to Predict the Need for Oxygenation Support and Intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep Learning in Medical Ultrasound Analysis: A Review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Penarrubia, L.; Verstraete, A.; Orkisz, M.; Davila, E.; Boussel, L.; Yonis, H.; Mezidi, M.; Dhelft, F.; Danjou, W.; Bazzani, A.; et al. Precision of CT-Derived Alveolar Recruitment Assessed by Human Observers and a Machine Learning Algorithm in Moderate and Severe ARDS. Intensive Care Med. Exp. 2023, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.P.P.L.; Kitamura, F.C.; Prado, G.F.; de Aguiar Kuriki, P.E.; Garcia, M.R.T. Machine Learning Model for Predicting Severity Prognosis in Patients Infected with COVID-19: Study Protocol from COVID-AI Brasil. PLoS ONE 2021, 16, e0245384. [Google Scholar] [CrossRef]
- Puhr-Westerheide, D.; Reich, J.; Sabel, B.O.; Kunz, W.G.; Fabritius, M.P.; Reidler, P.; Rübenthaler, J.; Ingrisch, M.; Wassilowsky, D.; Irlbeck, M.; et al. Article Sequential Organ Failure Assessment Outperforms Quantitative Chest Ct Imaging Parameters for Mortality Prediction in Covid-19 Ards. Diagnostics 2022, 12, 10. [Google Scholar] [CrossRef]
- Röhrich, S.; Hofmanninger, J.; Negrin, L.; Langs, G.; Prosch, H. Radiomics Score Predicts Acute Respiratory Distress Syndrome Based on the Initial CT Scan after Trauma. Eur. Radiol. 2021, 31, 5443–5453. [Google Scholar] [CrossRef]
- Sarkar, N.; Zhang, L.; Campbell, P.; Liang, Y.; Li, G.; Khedr, M.; Khetan, U.; Dreizin, D. Pulmonary Contusion: Automated Deep Learning-Based Quantitative Visualization. Emerg. Radiol. 2023, 30, 435–441. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wei, Y.; Li, M.; Zhang, Y.; Zhang, N.; Zhao, S.; Zeng, H.; Deng, W.; Huang, Z.; et al. Quantitative Analysis of Chest CT Imaging Findings with the Risk of ARDS in COVID-19 Patients: A Preliminary Study. Ann. Transl. Med. 2020, 8, 594. [Google Scholar] [CrossRef]
- Zhang, J.; Chng, C.B.; Chen, X.; Wu, C.; Zhang, M.; Xue, Y.; Jiang, J.; Chui, C.K. Detection and Classification of Pneumonia from Lung Ultrasound Images. In Proceedings of the 2020 5th International Conference on Communication, Image and Signal Processing, CCISP 2020, Chengdu, China, 13–15 November 2020. [Google Scholar]
- Baloescu, C.; Toporek, G.; Kim, S.; McNamara, K.; Liu, R.; Shaw, M.M.; McNamara, R.L.; Raju, B.I.; Moore, C.L. Automated Lung Ultrasound B-Line Assessment Using a Deep Learning Algorithm. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Wiedemann, N.; Cossio, M.; Buhre, C.; Brändle, G.; Leidermann, K.; Goulet, J.; Aujayeb, A.; Moor, M.; Rieck, B.; et al. Accelerating Detection of Lung Pathologies with Explainable Ultrasound Image Analysis. Appl. Sci. 2021, 11, 672. [Google Scholar] [CrossRef]
- Arntfield, R.; Vanberlo, B.; Alaifan, T.; Phelps, N.; White, M.; Chaudhary, R.; Ho, J.; Wu, D. Development of a Convolutional Neural Network to Differentiate among the Etiology of Similar Appearing Pathological b Lines on Lung Ultrasound: A Deep Learning Study. BMJ Open 2021, 11, e045120. [Google Scholar] [CrossRef] [PubMed]
- Erfanian Ebadi, S.; Krishnaswamy, D.; Bolouri, S.E.S.; Zonoobi, D.; Greiner, R.; Meuser-Herr, N.; Jaremko, J.L.; Kapur, J.; Noga, M.; Punithakumar, K. Automated Detection of Pneumonia in Lung Ultrasound Using Deep Video Classification for COVID-19. Inform. Med. Unlocked 2021, 25, 100687. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A. The Concept of “Baby Lung”. Intensive Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Caironi, P.; Pelosi, P.; Goodman, L.R. What Has Computed Tomography Taught Us about the Acute Respiratory Distress Syndrome? Am. J. Respir. Crit. Care Med. 2001, 164, 1701–1711. [Google Scholar] [CrossRef]
- Raghavendran, K.; Davidson, B.A.; Woytash, J.A.; Helinski, J.D.; Marschke, C.J.; Manderscheid, P.A.; Notter, R.H.; Knight, P.R. The Evolution Of Isolated Bilateral Lung Contusion from Blunt Chest Trauma In Rats: Cellular and Cytokine Responses. Shock 2005, 24, 132. [Google Scholar] [CrossRef]
- Ko, J.P.; Liu, G.; Klein, J.S.; Mossa-Basha, M.; Azadi, J.R. Pulmonary COVID-19: Multimodality Imaging Examples. RadioGraphics 2020, 40, 1893–1894. [Google Scholar] [CrossRef]
- Sheard, S.; Rao, P.; Devaraj, A. Imaging of Acute Respiratory Distress Syndrome. Respir. Care 2012, 57, 607. [Google Scholar] [CrossRef]
- Ball, L.; Vercesi, V.; Costantino, F.; Chandrapatham, K.; Pelosi, P. Lung Imaging: How to Get Better Look inside the Lung. Ann. Transl. Med. 2017, 5, 294. [Google Scholar] [CrossRef]
- Pierrakos, C.; Smit, M.R.; Hagens, L.A.; Heijnen, N.F.L.; Hollmann, M.W.; Schultz, M.J.; Paulus, F.; Bos, L.D.J. Assessment of the Effect of Recruitment Maneuver on Lung Aeration Through Imaging Analysis in Invasively Ventilated Patients: A Systematic Review. Front. Physiol. 2021, 12, 666941. [Google Scholar] [CrossRef]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef]
- Isabel García-Laorden, M.; Lorente, J.A.; Flores, C.; Slutsky, A.S.; Villar, J. Biomarkers for the Acute Respiratory Distress Syndrome: How to Make the Diagnosis More Precise. Ann. Transl. Med. 2017, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wang, J.; Shi, J.; Wu, Z.; Wang, Q.; Tang, Z.; He, K.; Shi, Y.; Shen, D. Review of Artificial Intelligence Techniques in Imaging Data Acquisition, Segmentation, and Diagnosis for COVID-19. IEEE Rev. Biomed. Eng. 2021, 14, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, N.; Ansari, S.; Lee, E.; Ward, K.R.; Sjoding, M.W. Collaborative Strategies for Deploying Artificial Intelligence to Complement Physician Diagnoses of Acute Respiratory Distress Syndrome. NPJ Digit. Med. 2023, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Pai, K.-C.; Chao, W.-C.; Huang, Y.-L.; Sheu, R.-K.; Chen, L.-C.; Wang, M.-S.; Lin, S.-H.; Yu, Y.-Y.; Wu, C.-L.; Chan, M.-C. Artificial Intelligence–Aided Diagnosis Model for Acute Respiratory Distress Syndrome Combining Clinical Data and Chest Radiographs. Digit. Health 2022, 8, 20552076221120316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; He, C.; Wang, D.; Shen, H.; Sun, J.; Gan, W.; Lu, J.; Liu, X. The Role of Imaging Techniques in Management of COVID-19 in China: From Diagnosis to Monitoring and Follow-Up. Med. Sci. Monit. 2020, 26, e924582. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Tso, C.F.; Green-Saxena, A.; Pellegrini, E.; Iqbal, Z.; Evans, D.; Hoffman, J.; Calvert, J.; Mao, Q.; Das, R. Semisupervised Deep Learning Techniques for Predicting Acute Respiratory Distress Syndrome From Time-Series Clinical Data: Model Development and Validation Study. JMIR Form. Res. 2021, 5, e28028. [Google Scholar] [CrossRef]
- Miller, P.R.; Croce, M.A.; Bee, T.K.; Qaisi, W.G.; Smith, C.P.; Collins, G.L.; Fabian, T.C. Ards after Pulmonary Contusion: Accurate Measurement of Contusion Volume Identifies High-Risk Patients. J. Trauma 2001, 51, 223–228, discussion 229–230. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Boyd Bigelow, D.; Petty, T.L.; Levine, B.E. Acute Respiratory Distress In Adults. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pesenti, A.; Rossi, G.P.; Vesconi, S.; Fox, U.; Kolobow, T.; Agostoni, A.; Pelizzola, A.; Langer, M.; Uziel, L.; et al. Treatment of Acute Respiratory Failure with Low-Frequency Positive-Pressure Ventilation and Extracorporeal Removal of CO2. Lancet 1980, 316, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kolobow, T.; Moretti, M.P.; Fumagalli, R.; Mascheroni, D.; Prato, P.; Chen, V.; Joris, M. Severe Impairment in Lung Function Induced by High Peak Airway Pressure during Mechanical Ventilation. Am. Rev. Respir. Dis. 1987, 135, 312–315. [Google Scholar]
- Hickling, K.G.; Henderson, S.J.; Jackson, R. Low Mortality Associated with Low Volume Pressure Limited Ventilation with Permissive Hypercapnia in Severe Adult Respiratory Distress Syndrome. Intensive Care Med. 1990, 16, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; D’Andrea, L.; Pelosi, P.; Vitale, G.; Pesenti, A.; Fumagalli, R. Regional Effects and Mechanism of Positive End-Expiratory Pressure in Early Adult Respiratory Distress Syndrome. JAMA 1993, 269, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung Recruitment in Patients with the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef]
- Chiumello, D.; Marino, A.; Brioni, M.; Menga, F.; Cigada, I.; Lazzerini, M.; Andrisani, M.C.; Biondetti, P.; Cesana, B.; Gattinoni, L. Visual Anatomical Lung CT Scan Assessment of Lung Recruitability. Intensive Care Med. 2013, 39, 66–73. [Google Scholar] [CrossRef]
- Chiumello, D.; Formenti, P.; Coppola, S. Lung Recruitment: What Has Computed Tomography Taught Us in the Last Decade? Ann. Intensive Care 2019, 9, 12. [Google Scholar] [CrossRef]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Munich, Germany, 5–9 October 2015; Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Volume 9351. [Google Scholar]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.M.P.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA—J. Am. Med. Assoc. 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Chiumello, D.; Busana, M.; Coppola, S.; Romitti, F.; Formenti, P.; Bonifazi, M.; Pozzi, T.; Palumbo, M.M.; Cressoni, M.; Herrmann, P.; et al. Physiological and Quantitative CT-Scan Characterization of COVID-19 and Typical ARDS: A Matched Cohort Study. Intensive Care Med. 2020, 46, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Modafferi, L.; Fratti, I. Risk Factors and Mortality in Elderly ARDS COVID-19 Compared to Patients without COVID-19. J. Clin. Med. 2022, 11, 5180. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Chiumello, D.; Busana, M.; Giola, E.; Palermo, P.; Pozzi, T.; Steinberg, I.; Roli, S.; Romitti, F.; Lazzari, S.; et al. Role of Total Lung Stress on the Progression of Early COVID-19 Pneumonia. Intensive Care Med. 2021, 47, 1130–1139. [Google Scholar] [CrossRef]
- Pozzi, T.; Fratti, I.; Tomarchio, E.; Bruno, G.; Catozzi, G.; Monte, A.; Chiumello, D.; Coppola, S. Early Time-Course of Respiratory Mechanics, Mechanical Power and Gas Exchange in ARDS Patients. J. Crit. Care 2024, 79, 154444. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Proposal for International Standardization of the Use of Lung Ultrasound for Patients with COVID-19. J. Ultrasound Med. 2020, 39, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-Care Lung Ultrasound in Patients with COVID-19—A Narrative Review. Anaesthesia 2020, 75, 1096–1104. [Google Scholar] [CrossRef]
- Kulkarni, S.; Down, B.; Jha, S. Point-of-Care Lung Ultrasound in Intensive Care during the COVID-19 Pandemic. Clin. Radiol. 2020, 75, 710.e1–710.e4. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Li, L.; Guan, J.; Liu, Z.; Han, J.; Chao, Y.; Wang, Z.; Yu, X. The Value of Lung Ultrasound Score on Evaluating Clinical Severity and Prognosis in Patients with Acute Respiratory Distress Syndrome. Chin. Crit. Care Med. 2015, 27, 579–584. [Google Scholar] [CrossRef]
- Haddam, M.; Zieleskiewicz, L.; Perbet, S.; Baldovini, A.; Guervilly, C.; Arbelot, C.; Noel, A.; Vigne, C.; Hammad, E.; Antonini, F.; et al. Lung Ultrasonography for Assessment of Oxygenation Response to Prone Position Ventilation in ARDS. Intensive Care Med. 2016, 42, 1546–1556. [Google Scholar] [CrossRef]
- Caltabeloti, F.P.; Monsel, A.; Arbelot, C.; Brisson, H.; Lu, Q.; Gu, W.J.; Zhou, G.J.; Auler, J.O.C.; Rouby, J.J. Early Fluid Loading in Acute Respiratory Distress Syndrome with Septic Shock Deteriorates Lung Aeration without Impairing Arterial Oxygenation: A Lung Ultrasound Observational Study. Crit. Care 2014, 18, R91. [Google Scholar] [CrossRef]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.J. Bedside Ultrasound Assessment of Positive End-Expiratory Pressure-Induced Lung Recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf, R.; Treml, B.; Rajsic, S. Lung Sonography in Critical Care Medicine. Diagnostics 2022, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiang, L.; Xi, X.; Jiang, Q.; Zhu, B.; Wang, M.; Xing, J.; Zhang, D. Prognostic Value of Extravascular Lung Water Assessed with Lung Ultrasound Score by Chest Sonography in Patients with Acute Respiratory Distress Syndrome. BMC Pulm. Med. 2015, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Soummer, A.; Perbet, S.; Brisson, H.; Arbelot, C.; Constantin, J.M.; Lu, Q.; Rouby, J.J. Ultrasound Assessment of Lung Aeration Loss during a Successful Weaning Trial Predicts Postextubation Distress. Crit. Care Med. 2012, 40, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Chiumello, D.; Umbrello, M.; Papa, G.F.S.; Angileri, A.; Gurgitano, M.; Formenti, P.; Coppola, S.; Froio, S.; Cammaroto, A.; Carrafiello, G. Global and Regional Diagnostic Accuracy of Lung Ultrasound Compared to CT in Patients with Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1599–1606. [Google Scholar] [CrossRef]
- Corradi, F.; Brusasco, C.; Pelosi, P. Chest Ultrasound in Acute Respiratory Distress Syndrome. Curr. Opin. Crit. Care 2014, 20, 98–103. [Google Scholar] [CrossRef]
- Volpicelli, G.; Mussa, A.; Garofalo, G.; Cardinale, L.; Casoli, G.; Perotto, F.; Fava, C.; Frascisco, M. Bedside Lung Ultrasound in the Assessment of Alveolar-Interstitial Syndrome. Am. J. Emerg. Med. 2006, 24, 689–696. [Google Scholar] [CrossRef]
- Corradi, F.; Via, G.; Forfori, F.; Brusasco, C.; Tavazzi, G. Lung Ultrasound and B-Lines Quantification Inaccuracy: B Sure to Have the Right Solution. Intensive Care Med. 2020, 46, 1081–1083. [Google Scholar] [CrossRef]
- Millington, S.J.; Arntfield, R.T.; Guo, R.J.; Koenig, S.; Kory, P.; Noble, V.; Mallemat, H.; Schoenherr, J.R. Expert Agreement in the Interpretation of Lung Ultrasound Studies Performed on Mechanically Ventilated Patients. J. Ultrasound Med. 2018, 37, 2659–2665. [Google Scholar] [CrossRef]
- Muse, E.D.; Topol, E.J. Guiding Ultrasound Image Capture with Artificial Intelligence. Lancet 2020, 396, 749. [Google Scholar] [CrossRef]
- Suri, J.; Agarwal, S.; Gupta, S.; Puvvula, A.; Viskovic, K.; Suri, N.; Alizad, A.; El-Baz, A.; Saba, L.; Fatemi, M.; et al. Systematic Review of Artificial Intelligence in Acute Respiratory Distress Syndrome for COVID-19 Lung Patients: A Biomedical Imaging Perspective. IEEE J. Biomed. Health Inform. 2021, 25, 4128–4139. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep Learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yu, D. Deep Learning: Methods and Applications. Found. Trends Signal Process. 2013, 7, 197–387. [Google Scholar] [CrossRef]
- Gibbons, R.C.; Magee, M.; Goett, H.; Murrett, J.; Genninger, J.; Mendez, K.; Tripod, M.; Tyner, N.; Costantino, T.G. Lung Ultrasound vs. Chest X-Ray Study for the Radiographic Diagnosis of COVID-19 Pneumonia in a High-Prevalence Population. J. Emerg. Med. 2021, 60, 615–625. [Google Scholar] [CrossRef]
- Pare, J.R.; Camelo, I.; Mayo, K.C.; Leo, M.M.; Dugas, J.N.; Nelson, K.P.; Baker, W.E.; Shareef, F.; Mitchell, P.M.; Schechter-Perkins, E.M. Point-of-Care Lung Ultrasound Is More Sensitive than Chest Radiograph for Evaluation of COVID-19. West. J. Emerg. Med. 2020, 21, 771–778. [Google Scholar] [CrossRef]


| Author, Year | Study Design | Aim | Endpoints | AI Model | Results |
|---|---|---|---|---|---|
| Nishiyama, 2020 [9] | single center retrospective | prediction of prognosis | To evaluate the relationship between CT volume of well-aerated lung region and prognosis in ARDS patients. | An automated lung volumetry software of lung CT scan to identify lung region volumes by CT attenuation densities. | Well-aerated lung regions showed a positive correlation with 28-day survival. Survival outcome was better for percentage of well-aerated lung region/predicted total lung capacity ≥40% than <40%. |
| Gresser, 2021 [10] | single center retrospective | prediction of prognosis | To assess the potential of AI-based CT assessment and clinical score to predict the need for ECMO therapy in COVID-19 ARDS. | CT software provides segmentation of lung lobes providing a CT severity score. | AI-based assessment of lung involvement on CT scans at hospital admission and the SOFA scoring, especially if combined, can be used as risk stratification tools for subsequent ECMO requirement. |
| Hermann, 2021 [11] | multicenter retrospective | alveolar recruitment | To compare the accuracy in the computation of recruitability on CT scan between automatic lung segmentation performed by a properly trained neural network and manual segmentation in ARDS and COVID-19. | A DL algorithm to automatically segment ARDS injured lungs to calculate the lung recruitment. | The AI segmentation showed the same degree of inaccuracy of the manual segmentation. The recruitability measured with manual and AI segmentation had a bias of +0.3% and −0.5% expressed as change in well-aerated tissue fraction. |
| Kang, 2021 [12] | single center retrospective | differential diagnosis | To train a DL classifier model to differentiate between COVID-19 and bacterial pneumonia based on automatic segmentation of lung and lesion regions. | A DL model with deformable convolution neural network architecture trained to differentiate lesion patches of COVID-19 from those of bacterial pneumonia on CT scan. | DL lung CT scan analysis with constructed lesion clusters achieved an accuracy of 91.2% for classifying COVID-19 and bacterial pneumonia patients. |
| Lanza, 2020 [13] | single center retrospective | prediction of prognosis | To test quantitative CT analysis using a semi-automated method as an outcome predictor in terms of need for oxygen support or intubation in COVID-19. | Quantitative CT analysis with a semi-automated segmentation algorithm that divides lungs into not aerated, poorly aerated, normally aerated and hyperinflated. | The amount of compromised lung volume can predict the need for oxygenation support (between 6–23% of compromised lung) and intubation (above 23%) and is a significant risk factor for in-hospital death. |
| Liu, 2020 [14] | single center retrospective | prediction of prognosis | To quantify pneumonia lesions by CT (% of ground-glass, semi-consolidation and consolidation volume) in the early days to predict progression to severe illness using AI algorithms in COVID-19. | CT quantitative analysis combines a fully convolutional network with adopting thresholding and morphological operations for segmentation of lung and pneumonia lesions. | CT features on day 0 and 4, and their changes from day 0 to day 4, showed predictive capability for severe illness within a 28-day follow up. CT quantification of pneumonia lesions can early and non-invasively predict the progression to severe illness. |
| Pennati, 2023 [2] | single center retrospective | alveolar recruitment | To develop and validate classifier models to identify patients with a high percentage of potentially recruitable lung from readily available clinical data and from a single CT scan quantitative analysis at ICU admission. | Four ML algorithms (Logistic regression, Support Vector Machine, Random Forest, XGboost) to predict lung recruitment starting from a single CT scan obtained at 5 cm H2O at ICU admission. | The use of the four ML algorithms based on a CT scan at 5 cm H2O were able to classify lung recruiter patients with similar AUC as the ML algorithm, based on the combination of lung mechanics, gas exchange and CT data. |
| Penarrubia, 2023 [15] | single center retrospective | alveolar recruitment | To assess both intra- and inter-observer smallest real difference exceeding measurement error of recruitment using both human and ML on low-dose CT scans acquired at 5 and 15 cm H2O of PEEP in ARDS. | ML lung segmentation algorithm on CT scan to compute alveolar recruitment at 5 and 15 cm H2O of PEEP. | Human–machine and human–human inter-observer measurement errors were similar, suggesting that ML segmentation algorithms are valid alternative to humans for quantifying alveolar recruitment on CT. |
| Lopes, 2021 [16] | multicenter retrospective (study protocol) | prediction of prognosis | To develop a ML based on clinical, radiological and epidemiological data to predict the severity prognosis (ICU admission, intubation) in COVID-19. | A ML model receives a lung CT as input and outputs the stratification of lung parenchyma, discerning regions of the lungs with different densities. | Study in progress |
| Puhr-Westerheide, 2022 [17] | single center retrospective | diagnosis | To compare AI-based quantitative CT severity score to SOFA score in predicting in-hospital mortality at ICU admission in COVID-19 ARDS patients. | AI-based lung injury assessment on CT scan for the diagnostic performance to predict in-hospital mortality. | CT severity score was not associated to in-hospital mortality prediction, whereas the SOFA score showed a significant association. |
| Röhrich, 2021 [18] | single center prospective | prediction of prognosis | To develop a ML model for the early ARDS prediction from the first CT scan of trauma patients at hospital admission. | A ML model with convolutional neural network (radiomics) approach to automatically delineate the lung at lung CT to predict future ARDS. | The ML model with radiomics score resulted in a higher AUC (0.79) compared to injury severity score (0.66) and abbreviated injury score of the thorax (0.68) in prediction of ARDS. The radiomics score achieved a sensitivity and a specificity of 0.80 and 0.76. |
| Sarkar, 2023 [19] | single center retrospective | diagnosis and prediction of prognosis | To train and validate DL models to quantify pulmonary contusion as a percentage of total lung volume and assess the relationship between automated Lung Contusion Index and relevant clinical outcomes (ICU LoS and mechanical ventilation time). | DL model for automated CT scan segmentation to quantify the percent lung involvement indexed to total lung volumes. | Automated Lung Contusion Index was associated with ARDS, longer ICU LoS and longer mechanical ventilation time. Automated Lung Contusion Index and clinical variables predicted ARDS with an AUC of 0.70, while automated Lung Contusion Index alone predicted ARDS with an AUC of 0.68. |
| Wang, 2020 [20] | retrospective study | diagnosis | To explore the relationship between the quantitative analysis results and the ARDS existence, using an automatic quantitative analysis model based on DL segmentation model in COVID-19. | DL model to provide an automatic quantitative analysis of infection regions on lung CT to assess their density and location. | The total volume and density of the lung infectious regions were not related to ARDS. The proportion of lesion density was associated with increased risk of ARDS in COVID-19. |
| Zhang, 2020 [21] | single center retrospective | diagnosis | To compare the performance of the three DL models and determine which model is more diagnostic. | Three DL models (VGG, Resnet and EfficientNet) are used to classify LUS images of pneumonia according to different clinical stages based on a self-made image dataset. | EfficientNet showed to be the best model providing the best accuracy for 3 and 4 clinical stages of pneumonia, with an accuracy of 94.62% and 91.18%, respectively. The best classification accuracy of 8 clinical features of pneumonia at LUS images was 82.75%. |
| Baloescu, 2020 [22] | single center retrospective | diagnosis | To test the DL algorithm to quantify the assessment of B lines in LUS images from a database of patients presenting at ED with dyspnea or chest pain and to compare the algorithm to expert human interpretation. | A DL model is trained and developed based on a dataset of LUS clips to assess presence/absence of B lines and severity classification. | The accuracy in detecting B lines was 94% with a kappa of 0.88; the accuracy of the severity assessment was 56% with a kappa of 0.65. |
| Born, 2021 [23] | multicenter retrospective | differential diagnosis | To compare different AI models for the differential diagnosis of COVID-19 pneumonia and bacterial pneumonia. | Five AI models (VGG, VGG-CAM, NASNetMobile, VGG-segement, Segment-Enc) are tested on a dataset of LUS images and videos of healthy controls and patients affected by COVID-19 and bacterial pneumonia and compared in terms of recall, precision, specificity and F1 scores. | Two models (VGG and VGG-CAMI) had an accuracy of 88 ± 5% in distinguishing COVID-19 pneumonia and bacterial pneumonia. |
| Arntfield, 2021 [24] | multicenter retrospective | differential diagnosis | To compare the DL model and the surveyed LUS-competent physicians in the ability of discriminating pathological LUS imaging | A DL convolutional neural network model is trained on LUS images with B lines to discriminate between COVID-19 ARDS, non-COVID ARDS and hydrostatic pulmonary edema and compared with surveyed LUS-competent physicians. | The DL model showed an ability to discriminate between COVID-19 ARDS (AUC 1.0), non-COVID ARDS (AUC 0.934) and pulmonary edema (AUC 1.0) better than physician ability (AUCs 0.697, 0.704, 0.967). |
| Ebadi, 2021 [25] | multicenter retrospective | differential diagnosis | To compare the DL classifier model against ground truth classification provided by expert radiologists and clinicians. | A DL method based on the Kinetics-I3D network. classifies an entire LUS scan, without the use of pre-processing or a frame-by-frame analysis, for automatic detection of ARDS features present in pneumonia and COVID-19 patients (A lines, B lines, consolidation and pleural effusion). | The DL model showed an accuracy of 90% and a precision score of 95% with the use of 5-fold cross validation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiumello, D.; Coppola, S.; Catozzi, G.; Danzo, F.; Santus, P.; Radovanovic, D. Lung Imaging and Artificial Intelligence in ARDS. J. Clin. Med. 2024, 13, 305. https://doi.org/10.3390/jcm13020305
Chiumello D, Coppola S, Catozzi G, Danzo F, Santus P, Radovanovic D. Lung Imaging and Artificial Intelligence in ARDS. Journal of Clinical Medicine. 2024; 13(2):305. https://doi.org/10.3390/jcm13020305
Chicago/Turabian StyleChiumello, Davide, Silvia Coppola, Giulia Catozzi, Fiammetta Danzo, Pierachille Santus, and Dejan Radovanovic. 2024. "Lung Imaging and Artificial Intelligence in ARDS" Journal of Clinical Medicine 13, no. 2: 305. https://doi.org/10.3390/jcm13020305