Prevalence, Severity of Extension, and Risk Factors of Gingivitis in a 3-Month Pregnant Population: A Multicenter Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Outcomes
2.3.1. Primary Outcome Measures
2.3.2. Secondary Outcome Measures
2.4. Procedures
2.4.1. Determination of Demographic and Behavioral Characteristics
2.4.2. Determination of the Prenatal Clinical Status
2.4.3. Determination of the Oral Clinical Parameters
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Clinical Parameters of Pregnant Women
3.2. Clinical Parameters of Pregnant Women
3.3. Prevalence of Gingivitis Cases in Pregnant Women
3.4. Distribution of No/Mild or Localized or Generalized Gingivitis Cases and Sociodemographic, Behavioral, and Clinical Variables
3.5. Association between Gingivitis Cases (>10%) and Sociodemographic, Behavioral, and Clinical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmstrup, P.; Plemons, J.; Meyle, J. Non-plaque-induced gingival diseases. J. Periodontol. 2018, 89 (Suppl. S1), S28–S45. [Google Scholar] [CrossRef] [PubMed]
- Katarzyńska-Konwa, M.; Obersztyn, I.; Trzcionka, A.; Mocny-Pachońska, K.; Mosler, B.; Tanasiewicz, M. Oral Status in Pregnant Women from Post-Industrial Areas of Upper Silesia in Reference to Occurrence of: Preterm Labors, Low Birth Weight and Type of Labor. Healthcare 2020, 8, 528. [Google Scholar] [CrossRef]
- Mesa, M.D.; Loureiro, B.; Iglesia, I.; Fernandez Gonzalez, S.; Llurba Olivé, E.; García Algar, O.; Solana, M.J.; Cabero Perez, M.J.; Sainz, T.; Martinez, L.; et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef] [PubMed]
- Massoni, R.S.D.S.; Aranha, A.M.F.; Matos, F.Z.; Guedes, O.A.; Borges, H.; Miotto, M.; Porto, A.N. Correlation of periodontal and microbiological evaluations, with serum levels of estradiol and progesterone, during different trimesters of gestation. Sci. Rep. 2019, 9, 11762. [Google Scholar] [CrossRef]
- Maybodi, F.R.; Haerian-Ardakani, A.; Vaziri, F.; Khabbazian, A.; Mohammadi-Asl, S. CPITN changes during pregnancy and maternal demographic factors ‘impact on periodontal health. Iran. J. Reprod. Med. 2015, 13, 107–112. [Google Scholar]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Paul, O.; Arora, P.; Mayer, M.; Chatterjee, S. Inflammation in Periodontal Disease: Possible Link to Vascular Disease. Front. Physiol. 2020, 11, 609614. [Google Scholar] [CrossRef]
- Lieske, B.; Makarova, N.; Jagemann, B.; Walther, C.; Ebinghaus, M.; Zyriax, B.-C.; Aarabi, G. Inflammatory Response in Oral Biofilm during Pregnancy: A Systematic Review. Nutrients 2022, 14, 4894. [Google Scholar] [CrossRef]
- Dommisch, H.; Staufenbiel, I.; Schulze, K.; Stiesch, M.; Winkel, A.; Fimmers, R.; Dommisch, J.; Jepsen, S.; Miosge, N.; Adam, K.; et al. Expression of antimicrobial peptides and interleukin-8 during early stages of inflammation: An experimental gingivitis study. J. Periodontal Res. 2015, 50, 836–845. [Google Scholar] [CrossRef]
- Erchick, D.J.; Rai, B.; Agrawal, N.K.; Khatry, S.K.; Katz, J.; LeClerq, S.C.; Reynolds, M.A.; Mullany, L.C. Oral hygiene, prevalence of gingivitis, and associated risk factors among pregnant women in Sarlahi District, Nepal. BMC Oral Health 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.K.; Parry, S. Periodontal Disease and Pregnancy Outcomes: Time to Move On? J. Women’s Health 2012, 21, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Nannan, M.; Xiaoping, L.; Ying, J. Periodontal disease in pregnancy and adverse pregnancy outcomes: Progress in related mechanisms and management strategies. Front. Med. 2022, 9, 963956. [Google Scholar] [CrossRef]
- Silk, H.; Douglass, A.B.; Douglass, J.M.; Silk, L. Oral health during pregnancy. Am. Fam. Physician 2008, 77, 1139–1144. [Google Scholar]
- Rahman, M.; Hassan, R.; Islam, Z.; Ahmad, M.S.; Alam, M.; Islam, K.M. Oral Health Status of Pregnant Women attended the Mothers and Children Welfare Center (MCWC) in Bangladesh. City Dent. Coll. J. 2013, 10, 1–4. [Google Scholar] [CrossRef]
- Rakchanok, N.; Amporn, D.; Yoshida, Y.; Harun-Or-Rashid, M.; Sakamoto, J. Dental caries and gingivitis among pregnant and non-pregnant women in Chiang Mai, Thailand. Nagoya J. Med. Sci. 2010, 72, 43–50. [Google Scholar] [PubMed]
- Wu, M.; Chen, S.-W.; Jiang, S.-Y. Relationship between Gingival Inflammation and Pregnancy. Mediat. Inflamm. 2015, 2015, 623427. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.; Berens, L. Periodontology and pregnancy: An overview of biomedical and epidemiological evidence. Periodontology 2000 2021, 87, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, V.T.; Manigandan, T.; Sarumathi, T.; Aarthi Nisha, V.; Amudhan, A. Dental Considerations in Pregnancy-A Critical Review on the Oral Care. J. Clin. Diagn. Res. 2013, 7, 948–953. [Google Scholar] [CrossRef]
- Gingivitis and Periodontitis: Overview; Institute for Quality and Efficiency in Health Care (IQWiG): Köln, Germany, 2020. Available online: https://www.informedhealth.org/ (accessed on 10 February 2023).
- Balan, P.; Brandt, B.; Chong, Y.; Crielaard, W.; Wong, M.; Lopez, V.; He, H.; Seneviratne, C. Subgingival Microbiota during Healthy Pregnancy and Pregnancy Gingivitis. JDR Clin. Transl. Res. 2021, 6, 343–351. [Google Scholar] [CrossRef]
- Gare, J.; Kanoute, A.; Meda, N.; Viennot, S.; Bourgeois, D.; Carrouel, F. Periodontal Conditions and Pathogens Associated with Pre-Eclampsia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 7194. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.T. Grand challenges in oral health and nutrition: We are what we eat. Front. Oral Health 2022, 3, 999817. [Google Scholar] [CrossRef] [PubMed]
- Uwambaye, P.; Munyanshongore, C.; Rulisa, S.; Shiau, H.; Nuhu, A.; Kerr, M.S. Assessing the association between periodontitis and premature birth: A case-control study. BMC Pregnancy Childbirth 2021, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.S.; Achimano, A.A. Prevalence and Risk Factors for Periodontal Disease Among Women Attending Antenatal Care in Public Hospitals, Southwest Ethiopia, 2022: A Multicenter Cross-Sectional Study. Clin. Cosmet. Investig. Dent. 2022, 14, 153–170. [Google Scholar] [CrossRef]
- Starzyńska, A.; Wychowański, P.; Nowak, M.; Sobocki, B.K.; Jereczek-Fossa, B.A.; Słupecka-Ziemilska, M. Association between Maternal Periodontitis and Development of Systematic Diseases in Offspring. Int. J. Mol. Sci. 2022, 23, 2473. [Google Scholar] [CrossRef]
- Kanoute, A.; Gare, J.; Meda, N.; Viennot, S.; Tramini, P.; Fraticelli, L.; Carrouel, F.; Bourgeois, D. Effect of Oral Prophylactic Measures on the Occurrence of Pre-Eclampsia (OP-PE) in High-Risk Pregnant Women: A Cluster Randomized Controlled Trial. Methods Protoc. 2021, 4, 61. [Google Scholar] [CrossRef]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The Joint WHO/FAO Expert Consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- CDC. Defining Adult Overweight and Obesity. Available online: https://www.cdc.gov/obesity/basics/adult-defining.html (accessed on 10 February 2023).
- Trombelli, L.; Farina, R.; Silva, C.; Tatakis, D.N. Plaque-induced gingivitis: Case definition and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S46–S73. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Caton, J.G.; Polson, A.M. The interdental bleeding index: A simplified procedure for monitoring gingival health. Compend. Contin. Educ. Dent. 1985, 6, 88, 90–2. [Google Scholar]
- Hofer, D.; Sahrmann, P.; Attin, T.; Schmidlin, P. Comparison of marginal bleeding using a periodontal probe or an interdental brush as indicators of gingivitis. Int. J. Dent. Hyg. 2011, 9, 211–215. [Google Scholar] [CrossRef]
- Bourgeois, D.; Saliasi, I.; Llodra, J.C.; Bravo, M.; Viennot, S.; Carrouel, F. Efficacy of interdental calibrated brushes on bleeding reduction in adults: A 3-month randomized controlled clinical trial. Eur. J. Oral Sci. 2016, 124, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Luigi, C.; Marco, M.; Gianluca, M.; Vittorio, C. A Proposed New Index for Clinical Evaluation of Interproximal Soft Tissues: The Interdental Pressure Index. Int. J. Dent. 2014, 2014, 345075. [Google Scholar] [CrossRef]
- Huang, S.; Li, R.; Zeng, X.; He, T.; Zhao, H.; Chang, A.; Bo, C.; Chen, J.; Yang, F.; Knight, R.; et al. Predictive modeling of gingivitis severity and susceptibility via oral microbiota. ISME J. 2014, 8, 1768–1780. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Al Khodor, S. Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 2021, 145, 103293. [Google Scholar] [CrossRef]
- Fan, W.; Liu, C.; Zhang, Y.; Yang, Z.; Li, J.; Huang, S. Epidemiology and associated factors of gingivitis in adolescents in Guangdong Province, Southern China: A cross-sectional study. BMC Oral Health 2021, 21, 311. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Baudet, A.; Veynachter, T.; Rousseau, H.; Anagnostou, F.; Jeanne, S.; Orti, V.; Thilly, N.; Clément, C.; Bisson, C. Perception of Gingival Bleeding by People and Healthcare Professionals: A Multicentre Study in an Adult French Population. Int. J. Environ. Res. Public Health 2020, 17, 5982. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Prasad, K.V. Distribution of dental plaque and gingivitis within the dental arches. J. Int. Med. Res. 2017, 45, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Li, S.; Wang, X.; Liu, J.; Li, X. The prevalence of gingivitis and related risk factors in schoolchildren aged 6–12 years old. BMC Oral Health 2022, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Kozak, U.; Lasota, A.; Chałas, R. Changes in Distribution of Dental Biofilm after Insertion of Fixed Orthodontic Appliances. J. Clin. Med. 2021, 10, 5638. [Google Scholar] [CrossRef]
- Oh, T.-J.; Eber, R.; Wang, H.-L. Periodontal diseases in the child and adolescent. J. Clin. Periodontol. 2002, 29, 400–410. [Google Scholar] [CrossRef]
- Opeodu, O.; Dosumu, E.; Arowojolu, M. Periodontal condition and treatment needs of some pregnant women in Ibadan, Nigeria. Ann. Med. Health Sci. Res. 2015, 5, 213–217. [Google Scholar] [CrossRef]
- Oyaro, B.; Lokken, E.; Alumera, H.; Hussein, S.; Richardson, B.; Mandaliya, K.; Jaoko, W.; Kinuthia, J.; Dimba, E.; Kemoli, A.; et al. Prevalence and correlates of periodontitis among Kenyan women planning to conceive. BMC Oral Health 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, W.; Li, J.; Cui, L.; Chen, Z.-J. Periodontal Disease and Adverse Neonatal Outcomes: A Systematic Review and Meta-Analysis. Front. Pediatr. 2022, 10, 799740. [Google Scholar] [CrossRef]
- Vogt, M.; Sallum, A.W.; Cecatti, J.G.; Morais, S.S. Factors associated with the prevalence of periodontal disease in low-risk pregnant women. Reprod. Health 2012, 9, 3. [Google Scholar] [CrossRef]
- Nuamah, I.; Annan, B.D. Periodontal status and oral hygiene practices of pregnant and non-pregnant women. East Afr. Med. J. 1998, 75, 712–714. [Google Scholar] [PubMed]
- Erchick, D.J.; Khatry, S.K.; Agrawal, N.K.; Katz, J.; LeClerq, S.C.; Rai, B.; Reynolds, M.A.; Mullany, L.C. Risk of preterm birth associated with maternal gingival inflammation and oral hygiene behaviours in rural Nepal: A community-based, prospective cohort study. BMJ Open 2020, 10, e036515. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.F.; Gilill, C.S.; DembÃ, J. Prevalence and Predictors of Periodontal Disease among Pregnant Women in Mali, West Africa. Ann. Med. Health Sci. Res. 2017, 7, 263–270. [Google Scholar]
- Piscoya, M.D.B.D.V.; Ximenes, R.A.D.A.; da Silva, G.M.; Jamelli, S.R.; Coutinho, S.B. Periodontitis-associated risk factors in pregnant women. Clinics 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Ramli, H.; Mohd-Dom, T.N.; Mohd-Said, S. Clinical benefits and adverse effects of siwak (S. persica) use on periodontal health: A scoping review of literature. BMC Oral Health 2021, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Carmagnola, D.; Pellegrini, G.; Malvezzi, M.; Canciani, E.; Henin, D.; Dellavia, C. Impact of Lifestyle Variables on Oral Diseases and Oral Health-Related Quality of Life in Children of Milan (Italy). Int. J. Environ. Res. Public Health 2020, 17, 6612. [Google Scholar] [CrossRef] [PubMed]
- Deghatipour, M.; Ghorbani, Z.; Ghanbari, S.; Arshi, S.; Ehdayivand, F.; Namdari, M.; Pakkhesal, M. Oral health status in relation to socioeconomic and behavioral factors among pregnant women: A community-based cross-sectional study. BMC Oral Health 2019, 19, 117. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715. [Google Scholar] [CrossRef]
- Lavigne, S.E. Evolving Evidence for Relationships between Periodontitis and Systemic Diseases: Position Paper from the Canadian Dental Hygienists Association. Can. J. Dent. Hyg. 2022, 56, 155–171. [Google Scholar]
- Abu-Shawish, G.; Betsy, J.; Anil, S. Is Obesity a Risk Factor for Periodontal Disease in Adults? A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 12684. [Google Scholar] [CrossRef]
- Balan, P.; He, H.-G.; Cao, F.; Wong, M.L.; Chong, Y.-S.; Lopez, V.; Soh, S.-E.; Seneviratne, C.J. Oral Health in Pregnant Chinese Women in Singapore: A Call to Go beyond the Traditional Clinical Care. Healthcare 2018, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Bushehab, N.M.E.; Sreedharan, J.; Reddy, S.; D’souza, J.; Abdelmagyd, H. Oral Hygiene Practices and Awareness of Pregnant Women about the Effects of Periodontal Disease on Pregnancy Outcomes. Int. J. Dent. 2022, 2022, 5195278. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, R.; Cheng, R.; Xu, T.; Zhang, T.; Hong, X.; Zhao, X.; Wu, Y.; Cheng, L.; Hu, T. Gingival bleeding and calculus among 12-year-old Chinese adolescents: A multilevel analysis. BMC Oral Health 2020, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.; Bravo, M.; Llodra, J.-C.; Inquimbert, C.; Viennot, S.; Dussart, C.; Carrouel, F. Calibrated interdental brushing for the prevention of periodontal pathogens infection in young adults—A randomized controlled clinical trial. Sci. Rep. 2019, 9, 15127. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, P.C.; Krom, B.P.; van der Veen, M.H. Sex Steroid Hormones as a Balancing Factor in Oral Host Microbiome Interactions. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Ye, C.; Kapila, Y. Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontology 2000 2021, 87, 276–281. [Google Scholar] [CrossRef]
- Bao, J.; Huang, X.; Wang, L.; He, Y.; Rasubala, L.; Ren, Y.-F. Clinical Practice Guidelines for Oral Health Care during Pregnancy: A Systematic Evaluation and Summary Recommendations for General Dental Practitioners. Quintessence Int. 2022, 53, 362–373. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Van Der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S71–S76. [Google Scholar] [CrossRef]
- Seminario, A.L.; DeRouen, T.; Cholera, M.; Liu, J.; Phantumvanit, P.; Kemoli, A.; Castillo, J.; Pitiphat, W. Mitigating Global Oral Health Inequalities: Research Training Programs in Low- and Middle-Income Countries. Ann. Glob. Health 2020, 86, 141. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L. Dental plaque-induced gingival conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Lieff, S.; Boggess, K.A.; Murtha, A.P.; Jared, H.; Madianos, P.N.; Moss, K.; Beck, J.; Offenbacher, S. The Oral Conditions and Pregnancy Study: Periodontal Status of a Cohort of Pregnant Women. J. Periodontol. 2004, 75, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; Rivero-Blanco, T.; Leon-Rios, X.; Exposito-Ruiz, M.; Pérez-Castillo, I.; Aguilar-Cordero, M.J. Oral and general health conditions involved in periodontal status during pregnancy: A prospective cohort study. Arch. Gynecol. Obstet. 2022. [Google Scholar] [CrossRef]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Periodontol. 2018, 89 (Suppl. S1), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; La Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Herath, T.D.K.; Wang, Y.; Seneviratne, C.J.; Darveau, R.P.; Wang, C.-Y.; Jin, L. The expression and regulation of matrix metalloproteinase-3 is critically modulated by Porphyromonas gingivalis lipopolysaccharide with heterogeneous lipid A structures in human gingival fibroblasts. BMC Microbiol. 2013, 13, 73. [Google Scholar] [CrossRef]
- Tenelanda-López, D.; Valdivia-Moral, P.; Castro-Sánchez, M. Eating Habits and Their Relationship to Oral Health. Nutrients 2020, 12, 2619. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, H.-S.; Lee, D.; Kim, K.; Kim, Y.-H. Association between Four Dietary Patterns and the Risk of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4362. [Google Scholar] [CrossRef]
- Liu, Y. The relationship between lifestyle and self-reported oral health among American adults. Int. Dent. J. 2014, 64, 46–51. [Google Scholar] [CrossRef]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin D in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020, 55, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Jin, K.; Dong, X.; Qiu, S.; Han, X.; Yu, Y.; Bai, D. Association of Diet-Related Systemic Inflammation with Periodontitis and Tooth Loss: The Interaction Effect of Diabetes. Nutrients 2022, 14, 4118. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef]



| Characteristics | Overall (N = 220) |
|---|---|
| Age (years) | |
| Mean ± SD | 23.6 ± 4.6 |
| Median [IQR] | 23 [20.0–26.0] |
| Pregnancy age (weeks) | |
| Mean ± SD | 12.3 ±0.7 |
| Median [IQR] | 12 [12.0–13.0] |
| Professional activity, n/N (%) | |
| No | 139/220 (63.2) |
| Yes | 81/220 (36.8) |
| Residence, n/N (%) | |
| Urban | 209/220 (95.0%) |
| Rural | 11/220 (5.0%) |
| BMI (kg/m2), | |
| Mean ± SD | 23.1 ± 4.5 |
| Median [IQR] | 22.6 [22.2–25.1] |
| Education, n/N (%) | |
| No | 47/220 (21.4%) |
| 1–6 years | 34/220 (15.5%) |
| 7–12 years | 59/220 (27.0%) |
| ≥13 years | 79/220 (36.1%) |
| Fruits and vegetables consumption, n/N (%) | |
| <5 portions by day | 161/220 (74.5) |
| ≥5 portions by day | 55/220 (25.5) |
| Physical activity, n/N (%) | |
| No <1500 metabolic equivalent of task/week | 55/220 (24.8) |
| Yes ≥1500 metabolic equivalent of task/week | 165/220 (75.2) |
| Sedentary behavior, n/N (%) | |
| Active | 11/220 (5.0) |
| Sedentary | 209/220 (95) |
| Toothbrushing frequency, n/N (%) | |
| 1/day | 66/220 (30.0) |
| ≥2/day | 154/220 (70.0) |
| Characteristics | Overall (N = 220) |
|---|---|
| Caries free, n/N (%) | |
| Yes | 68/220 (68.9) |
| No | 151/220 (31.1) |
| Decayed teeth | |
| Mean ± SD | 1.8 ± 2.5 |
| Median [IQR] | 1.0 [0.0–3.0] |
| Missing teeth | |
| Mean ± SD | 0.7 ± 1.3 |
| Median [IQR] | 0.0 [0.0–1.0] |
| Filled teeth | |
| Mean ± SD | 0.1 ± 0.5 |
| Median [IQR] | 0.0 [0.0–0.0] |
| DMFT (Decayed, Missing, Filled teeth) | |
| Mean ± SD | 2.7 ± 3.1 |
| Median [IQR] | 2.0 [0.0–4.0] |
| Plaque index | |
| Mean ± SD | 0.5 ± 0.5 |
| Median [IQR] | 0.4 [0.1–0.8] |
| Gingival index | |
| Mean ± SD | 0.2 ± 0.4 |
| Median [IQR] | 0.1 [0.0–0.3] |
| Pocket depth (mm) | |
| Mean ± SD | 2.2 ± 0.6 |
| Median [IQR] | 2.2 [1.8–2.6] |
| Clinical attachment level (mm) | |
| Mean ± SD | 2.0 ± 1.1 |
| Median [IQR] | 2.3 [1.4–2.7] |
| Bleeding on interdental brushing (%) | |
| Mean ± SD | 58.5 ± 34.0 |
| Median [IQR] | 66.7 [28.8–92.1] |
| Characteristics | No/Mild N = 26 | Localized Gingivitis N = 33 | Generalized Gingivitis N = 161 | p Value |
|---|---|---|---|---|
| Age (years) | 21.8 (±3.7) | 24.0 (±4.0) | 23.8 (±5.0) | 0.060 a |
| Pregnancy age (weeks) | 12.4 ± 0.7 | 12.3 ± 0.8 | 12.3 ± 0.7 | 0.516 a |
| Professional activity, n/N (%) | ||||
| No | 7 (26.9) | 16 (48.5) | 103 (64.0) | 0.216 b |
| Yes | 19 (73.1) | 17 (51.5) | 58 (36.0) | |
| Residence, n/N (%) | 0.457 b | |||
| Urban | 26 (100) | 31 (93.9) | 152 (94.4) | |
| Rural | - | 2 (6.1) | 9 (5.6) | |
| BMI (kg/m2) | 23.4 ± 4.3 | 21.2 ± 3.3 | 23.4 ± 4.6 | 0.030 a |
| Education, n/N (%) | 0.246 b | |||
| No | 10 (38.5) | 5 (15.2) | 33 (20.0) | |
| 1–6 years | 3 (11.5) | 8 (24.2) | 23 (14.4) | |
| 7–12 years | 7 (26.9) | 9 (27.3) | 43 (26.9) | |
| ≥13 years | 6 (23.1) | 11 (33.3) | 62 (38.8) | |
| Fruits and vegetables consumption | 0.108 b | |||
| <5 portions/day | 15 (57.7) | 26 (78.1) | 122 (76.6) | |
| ≥5 portions/day | 11 (42.3) | 7 (21.9) | 37 (23.4) | |
| Physical activity | 0.699 b | |||
| Yes | 8 (30.8) | 7 (21.2) | 40 (24.8) | |
| No | 18 (69.2) | 26 (78.8) | 121 (75.2) | |
| Sedentary behavior | 0.133 b | |||
| Active | 3 (11.5) | - | 8 (5.0) | |
| Sedentary | 23 (88.5) | 33 (100.0) | 151 (95.0) | |
| Toothbrushing frequency | 0.333 b | |||
| 1/day | 11 (42.3) | 10 (30.3) | 45 (28.0) | |
| ≥2/day | 15 (57.7) | 23 (69.7) | 116 (72.0) | |
| Caries free | 0.994 b | |||
| Yes | 8 (30.8) | 10 (30.3) | 50 (31.2) | |
| No | 18 (69.2) | 23 (69.7) | 110 (68.8) | |
| Plaque index | 1.44 ± 0.50 | 1.89 ± 0.59 | 2.35 ± 0.48 | <0.001 a |
| CAL (mm) | 0.52 ± 0.94 | 1.27 ± 1.33 | 2.33 ± 0.85 | <0.001 a |
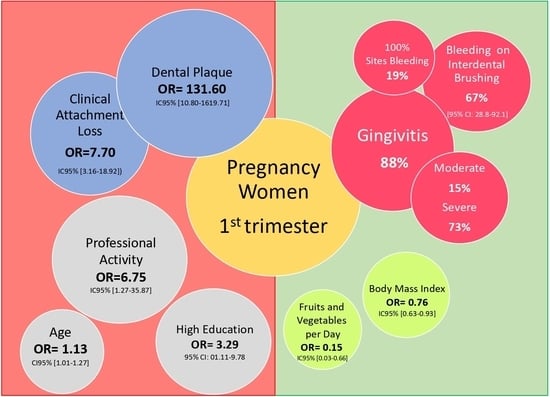
| Characteristic | Healthy (n = 26) | Gingivitis (n = 194) | Unadjusted OR [95% CI] | p Value | Adjusted OR [95% CI] | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 21.8 ± 3.7 | 21.1 ± 3.1 | 1.13 [1.01–1.27] | 0.03 | 1.14 [0.98–1.32] | 0.09 |
| Pregnancy age (weeks) | 12.4 ± 0.7 | 12.3 ± 0.7 | 0.76 [0.45–1.3] | 0.32 | ||
| Professional activity, n/N (%) | ||||||
| No | 7 (26.9) | 119 (61.3) | 1 | |||
| Yes | 19 (73.1) | 95 (38.7) | 1.68 [0.67–4.18] | 0.27 | 6.75 [1.27–35.87] | 0.02 |
| Residence, n/N (%) | ||||||
| Urban | 26 (100) | 183 (94.3) | 1 | |||
| Rural | - | 11 (5.7) | 6016402.22 [0.00-Inf] | 0.99 | ||
| BMI (kg/m2) | 23.9 ± 4.7 | 22.9 ± 4.7 | 0.98 [0.90–1.08] | 0.90 | 0.76 [0.63–0.93] | 0.01 |
| Education, n/N (%) | ||||||
| No | 10 (38.5) | 38 (19.1) | 1 | |||
| 1–6 years | 3 (11.5) | 31 (15.5) | 2.80 [0.70–11.02] | 0.14 | ||
| 7–12 years | 7 (26.9) | 52 (26.8) | 2.01 [0.70–5.75] | 0.19 | ||
| ≥13 years | 6 (23.1) | 73 (37.6) | 3.29 [01.11–9.78] | 0.03 | ||
| Fruits and vegetables consumption | ||||||
| <5 portions/day | 15 (57.7) | 148 (76.3) | 1 | |||
| ≥5 portions/day | 11 (42.3) | 46 (23.7) | 0.41 [0.18–0.98] | 0.04 | 0.15 [0.03–0.66] | 0.01 |
| Physical activity | ||||||
| Yes | 18 (69.2) | 147 (75.7) | 1 | |||
| No | 8 (30.8) | 47 (24.3) | 1.39 [0.57–3.39] | 0.47 | ||
| Sedentary behavior | ||||||
| Active | 3 (11.5) | 10 (5.1) | 1 | |||
| Sedentary | 23 (88.5) | 184 (94.9) | 3.0 [0.74–12.06] | 0.12 | 0.53 [0.05–5.81] | 0.92 |
| Toothbrushing frequency | ||||||
| 1/day | 11 (42.3) | 55 (28.4) | 1 | |||
| ≥2/day | 15 (57.7) | 139 (71.6) | 1.86 [0.80–4.31] | 0.14 | 0.90 [0.26–3.10] | 0.82 |
| Caries free | ||||||
| Yes | 18 (69.2) | 134 (69.1) | 1 | |||
| No | 8 (30.8) | 60 (30.9) | 1.01 [0.42–2.46] | 0.97 | 0.95 [0.25–3.60] | 0.94 |
| Plaque index | 0.19 ± 0.25 | 0.59 ± 0.48 | 27.66 [4.81–159.17] | <0.001 | 131.6 [10.80–1619.71] | <0.001 |
| CAL (mm) | 0.52 ± 0.94 | 1.95 ± 1.06 | 3.71 [2.34–5.87] | <0.001 | 7.7 [3.16–18.92] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gare, J.; Kanoute, A.; Orsini, G.; Gonçalves, L.S.; Ali Alshehri, F.; Bourgeois, D.; Carrouel, F. Prevalence, Severity of Extension, and Risk Factors of Gingivitis in a 3-Month Pregnant Population: A Multicenter Cross-Sectional Study. J. Clin. Med. 2023, 12, 3349. https://doi.org/10.3390/jcm12093349
Gare J, Kanoute A, Orsini G, Gonçalves LS, Ali Alshehri F, Bourgeois D, Carrouel F. Prevalence, Severity of Extension, and Risk Factors of Gingivitis in a 3-Month Pregnant Population: A Multicenter Cross-Sectional Study. Journal of Clinical Medicine. 2023; 12(9):3349. https://doi.org/10.3390/jcm12093349
Chicago/Turabian StyleGare, Jocelyne, Aida Kanoute, Giovanna Orsini, Lucio Souza Gonçalves, Fahad Ali Alshehri, Denis Bourgeois, and Florence Carrouel. 2023. "Prevalence, Severity of Extension, and Risk Factors of Gingivitis in a 3-Month Pregnant Population: A Multicenter Cross-Sectional Study" Journal of Clinical Medicine 12, no. 9: 3349. https://doi.org/10.3390/jcm12093349