The Gastropack Access System as a Model to Access Gastroenterology Services for Gastroscopy Appropriateness in Patients with Upper Gastrointestinal Symptoms: A Comparison with the Open Access System
Abstract
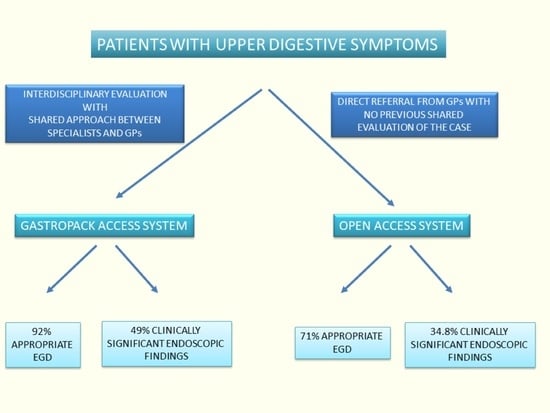
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Groups
2.3. Statistical Analyses
3. Results
3.1. Study Populations
3.2. Appropriateness of EGD Indication
3.3. Clinically Significant Endoscopic Findings (CSEF)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Gastropack System Study Group
- 1
- Department of Gastroenterology and Interventional Endoscopy, AUSL Bologna Bellaria, Maggiore Hospital Bologna, 40133 Bologna, Italy
- 5
- Department of Medical and Surgical Sciences, University of Bologna, 40126 Bologna, Italy
- 6
- Unit of Semeiotics, Liver and Alcohol-related Diseases, IRCCS Azienda Ospedaliero, Universitaria di Bologna, 40126 Bologna, Italy
- 9
- Department of Primary Care, Distretto Appennino Bolognese, AUSL Bologna, 40124 Bologna, Italy
References
- ASGE Standards of Practice Committee; Chandrasekhara, V.; Eloubeidi, M.A.; Bruining, D.H.; Chathadi, K.; Faulx, A.L.; Fonkalsrud, L.; Khashab, M.A.; Lightdale, J.R.; Muthusamy, V.R.; et al. Open-access endoscopy. Gastrointest. Endosc. 2015, 81, 1326–1329. [Google Scholar] [CrossRef]
- Pike, I.M. Open-access endoscopy. Gastrointest. Endosc. Clin. N. Am. 2006, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Keren, D.; Rainis, T.; Stermer, E.; Lavy, A. A nine-year audit of open-access upper gastrointestinal endoscopic procedures: Results and experience of a single centre. Can. J. Gastroenterol. 2011, 25, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Caselli, M.; Parente, F.; Palli, D.; Covacci, A.; Alvisi, V.; Gasbarrini, G.; Porro, G.B.; Cervia Working Group. “Cervia Working Group Report”: Guidelines on the diagnosis and treatment of Helicobacter pylori infection. Dig. Liver Dis. 2001, 33, 75–80. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Early, D.S.; Ben-Menachem, T.; Decker, G.A.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Fukami, N.; Hwang, J.H.; Jain, R.; et al. Appropriate use of GI endoscopy. Gastrointest. Endosc. 2012, 75, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, E.; Hassan, C.; Marmo, R.; Zullo, A.; Annibale, B. Appropriateness of the indication for upper endoscopy: A meta-analysis. Dig. Liver Dis. 2010, 42, 122–126. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.W.; Albasri, A.; Nicholson, B.D.; Perera, R.; Aronson, J.K.; Roberts, N.; Heneghan, C. Overtesting and undertesting in primary care: A systematic review and meta-analysis. BMJ Open 2018, 8, e018557. [Google Scholar] [CrossRef] [PubMed]
- Kisloff, B.; Peele, P.B.; Sharam, R.; Slivka, A. Quality of patient referral information for open-access endoscopic procedures. Gastrointest. Endosc. 2006, 64, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Bersani, G.; Buri, L.; Zullo, A.; Anti, M.; Bianco, M.A.; Di Giulio, E.; Ficano, L.; Morini, S.; Di Matteo, G.; et al. Appropriateness of upper-GI endoscopy: An Italian survey on behalf of the Italian Society of Digestive Endoscopy. Gastrointest. Endosc. 2007, 65, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Broe, M.; Barry, M.; Patchett, S.; Hill, A.D. Evaluating the clinical efficacy and cost effectiveness of direct access endoscopy. Surgeon 2013, 11, 304–308. [Google Scholar] [CrossRef]
- Chan, Y.M.; Goh, K.L. Appropriateness and diagnostic yield of EGD: A prospective study in a large Asian hospital. Gastrointest. Endosc. 2004, 59, 517–524. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Panganamamula, K.; Nieto, J.M.; Murad, M.H.; Keswani, R.N.; Shaukat, A.; Day, L.W. Interventions to improve the performance of upper GI endoscopy quality indicators. Gastrointest. Endosc. 2022, 96, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, F.; Repond, C.; Müllhaupt, B.; Vader, J.P.; Burnand, B.; Schneider, C.; Pache, I.; Thorens, J.; Rey, J.P.; Debosset, V.; et al. Is the diagnostic yield of upper GI endoscopy improved by the use of explicit panel-based appropriateness criteria? Gastrointest. Endosc. 2000, 52, 333–341. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.J.; Lantinga, M.A.; Drenth, J.P. Prevention of overuse: A view on upper gastrointestinal endoscopy. World J. Gastroenterol. 2019, 25, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Banait, G.; Sibbald, B.; Thompson, D.; Summerton, C.; Hann, M.; Talbot, S. Modifying dyspepsia management in primary care: A cluster randomised controlled trial of educational outreach compared with passive guideline dissemination. Br. J. Gen. Pract. 2003, 53, 94–100. [Google Scholar]
- Cardin, F.; Zorzi, M.; Bovo, E.; Guerra, C.; Bandini, F.; Polito, D.; Bano, F.; Grion, A.M.; Toffanin, R. Effect of implementation of a dyspepsia and Helicobacter pylori eradication guideline in primary care. Digestion 2005, 72, 1–7. [Google Scholar] [CrossRef]
- Elwyn, G.; Owen, D.; Roberts, L.; Wareham, K.; Duane, P.; Allison, M.; Sykes, A. Influencing referral practice using feedback of adherence to NICE guidelines: A quality improvement report for dyspepsia. Qual. Saf. Health Care 2007, 16, 67–70. [Google Scholar] [CrossRef]
- Niv, Y.; Dickman, R.; Levi, Z.; Neumann, G.; Ehrlich, D.; Bitterman, H.; Dreiher, J.; Cohen, A.; Comaneshter, D.; Halpern, E. Establishing an integrated gastroenterology service between a medical center and the community. World J. Gastroenterol. 2015, 21, 2152–2158. [Google Scholar] [CrossRef]
- Greenwood-Lee, J.; Jewett, L.; Woodhouse, L.; Marshall, D.A. A categorisation of problems and solutions to improve patient referrals from primary to specialty care. BMC Health Serv. Res. 2018, 18, 986. [Google Scholar] [CrossRef]
- Shaw, S.R.R.; Rumbold, B. What Is Integrated Care? A Research Report. 2011. Available online: https://www.nuffieldtrust.org.uk/files/2017-01/what-is-integrated-care-report-web-final.pdf (accessed on 24 October 2021).
- Nicholson, C.; Jackson, C.; Marley, J. A governance model for integrated primary/secondary care for the health-reforming first world—Results of a systematic review. BMC Health Serv. Res. 2013, 13, 528. [Google Scholar] [CrossRef]
- Health Mo. Piano Nazionale Di Governo Delle Liste Di Attesa per Il Triennio 2019–2021. 2019. Available online: https://www.salute.gov.it/portale/documentazione/ (accessed on 24 October 2021).
- Rossi, A.; Bersani, G.; Ricci, G.; DeFabritiis, G.; Pollino, V.; Suzzi, A.; Gorini, B.; Alvisi, V. ASGE guidelines for the appropriate use of upper endoscopy: Association with endoscopic findings. Gastrointest. Endosc. 2002, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.J.; Fennerty, M.B.; Bergman, J.J. Less Is More: A Minimalist Approach to Endoscopy. Gastroenterology 2018, 154, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 566–584. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar]
- Di Giulio, E.; Hassan, C.; Pickhardt, P.J.; Zullo, A.; Laghi, A.; Kim, D.H.; Iafrate, F. Cost-effectiveness of upper gastrointestinal endoscopy according to the appropriateness of the indication. Scand J. Gastroenterol. 2009, 44, 491–498. [Google Scholar] [CrossRef]
- Manes, G.; Balzano, A.; Marone, P.; Lioniello, M.; Mosca, S. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy system: A prospective observational study based on the Maastricht guidelines. Aliment Pharmacol. Ther. 2002, 16, 105–110. [Google Scholar] [CrossRef]
- Tahir, M. Appropriateness of Upper Gastrointestinal Endoscopy: Will the Diagnostic Yield Improve by the use of American Society of Gastroenterology Guidelines? Euroasian J. Hepatogastroenterol. 2016, 6, 143–148. [Google Scholar] [CrossRef]
- Leal, C.; Almeida, N.; Silva, M.; Santos, A.; Vasconcelos, H.; Figueiredo, P. Appropriateness of Endoscopic Procedures: A Prospective, Multicenter Study. GE Port. J. Gastroenterol. 2021, 29, 5–12. [Google Scholar] [CrossRef]
- Zullo, A.; Manta, R.; De Francesco, V.; Fiorini, G.; Hassan, C.; Vaira, D. Diagnostic yield of upper endoscopy according to appropriateness: A systematic review. Dig. Liver Dis. 2019, 51, 335–339. [Google Scholar] [CrossRef]
- Mourad, F.H.; Taylor, T.M.; Fairclough, P.D.; Farthing, M.J. General practitioner access to gastroscopy: Is ‘censorship’ valuable? Br. J. Gen. Pract. 1998, 48, 1165–1166. [Google Scholar]




| GAS Group (1424 Cases) | OAS Group (874 Cases) | p | |
|---|---|---|---|
| Demographic characteristic of patients | |||
| Age (median [IQR]), years | 61 [49–72] | 58 [45–68] | <0.01 |
| Gender (n, %), M/F | 571 (40)/853 (60) | 383 (44)/491 (56) | 0.043 |
| Appropriate indication | 1312 (92%) | 618 (71%) | <0.001 |
| Upper pesistent symptoms | 75 (5.7) | 97 (15.7) | <0.001 |
| Upper symptoms suggesting organic disease or in pts > 45 yrs | 445 (33.9) | 154 (24.9) | <0.001 |
| Esophageal reflux symptoms persistent or recurrent | 451 (34.4) | 204 (33.0) | Ns |
| Portal hypertension evaluation | 7 (0.5) | 10 (1.6) | 0.02 |
| Active or recent bleeding | 51 (3.9) | 27 (4.4) | Ns |
| Chronic blood loss or iron deficiency anemia | 99 (7.5) | 21 (3.4) | <0.001 |
| Tissue sampling | 5 (0.4) | 23 (3.7) | <0.001 |
| Dysphagia | 68 (7.2) | 19 (3.1) | 0.045 |
| Persistent vomiting | 47 (3.6) | 16 (2.6) | Ns |
| Surveillance of premalignant conditions | 64 (4.9) | 33 (5.3) | Ns |
| Not appropriate indication | 112 (8%) | 256 (29%) | <0.001 |
| Symptoms considered functional | 48 (33.0) | 127 (49.6) | <0.001 |
| Surveillance of healed benign lesions | 6 (5.4) | 40 (15.6) | 0.006 |
| GAS n (%) 252 pts | OAS n (%) 220 pts | p | |
|---|---|---|---|
| Appropriate indication | 205 (81) | 125 (57) | <0.001 |
| Upper persistent symptoms despite an appropriate trial of therapy | 9 (4.4) | 31 (24.8) | <0.001 |
| Upper symptoms suggesting organic disease or pts > 45 years | 86 (42) | 19 (15.2) | <0.001 |
| Esophageal reflux symptom persistent or recurrent | 57 (28) | 35 (28) | Ns |
| Portal hypertension evaluation | - | - | |
| Active or recent bleeding | 8 (3.9) | 8 (6.4) | Ns |
| Chronic blood loss or iron deficiency anemia | 20 (9.8) | 1 (0.8) | <0.001 |
| Sampling of tissue | - | 7 (5.6) | 0.05 |
| Dysphagia | 12 (5.9) | 4 (3.2) | Ns |
| Persistent vomiting | 12 (5.9) | 7 (5.6) | Ns |
| Surveillance of premalignant condition * | 1 (0.5) | 6 (4.8) | 0.04 |
| Not appropriate indication | 47 (19) | 95 (43) | <0.001 |
| Symptoms considered functional | 38 (80) | 77 (81.1) | Ns |
| Surveillance of healed benign lesions | 2 (4.3) | 10 (18.6) | 0.01 |
| GAS n (%) 1172 pts | OAS n (%) 654 pts | p | |
|---|---|---|---|
| Appropriate indication | n = 1107 (95) | n = 493 (75) | <0.001 |
| Upper pesistent symptoms despite an appropriate trial of therapy | 66 (6%) | 66 (13.4) | <0.001 |
| Upper symptoms suggesting organic disease or pts > 45 yrs | 359 (32.4) | 135 (27.4) | 0.04 |
| Esophageal reflux symptom persistent or recurrent | 394 (35.6) | 169 (34.3) | Ns |
| Portal hypertension evaluation | 7 (0.6) | 10 (2) | 0.01 |
| Active or recent bleeding | 43 (3.9) | 19 (3.9) | Ns |
| Chronic blood loss or iron deficiency anemia | 79 (7.1) | 20 (4.1) | 0.01 |
| Sampling of tissue | 5 (0.5) | 16 (3.2) | <0.001 |
| Dysphagia | 56 (5.1) | 15 (3) | Ns |
| Persistent vomiting | 35 (3.2) | 9 (1.8) | Ns |
| Surveillance of premalignant condition * | 63 (5.7) | 27 (5.5) | Ns |
| Not appropriate indication | 65 (5) | n = 161 (25) | <0.001 |
| Symptoms considered functional | 10 (15) | 50 (31.1) | 0.001 |
| Surveillance of healed benign lesions | 4 (6.2) | 30 (18.6) | 0.011 |
| GAS Group | OAS Group | p | GAS Group | OAS Group | |||||
|---|---|---|---|---|---|---|---|---|---|
| CSEF | 697/1424 (49%) | 105/302 (34.8%) | <0.001 | Appropriate Indication 645/697 (92.5%) | Inappropriate Indication 52/697 (7.5%) | p | Appropriate Indication 75/105 (71.4%) | Inappropriate Indication 30/105 (28.6%) | p |
| Erosive gastroduodenitis | 143 (20%) | 21 (20%) | Ns | 134 (21%) | 9 (17%) | Ns | 12 | 9 | Ns |
| Erosive esophagitis | 184 (26%) | 61 (58.1%) | <0.001 | 170 (26%) | 14 (26%) | Ns | 41 | 20 | Ns |
| Gastric ulcer | 20 (2%) | 2 (1.9%) | Ns | 20 (3%) | 0 | Ns | 2 | 0 | Ns |
| Duodenal ulcer | 24 (3%) | 1 (1) | Ns | 22 (3%) | 2 (4%) | Ns | 1 | 0 | Ns |
| Bleeding lesions | 2 (0.2%) | 1 (1) | Ns | 2 (3%) | 0 | Ns | 0 | 1 | Ns |
| Gastric preneoplastic lesions | 217 (31%) | 2 (1.9) | <0.001 | 198 (30%) | 19 (36%) | Ns | 2 | 0 | Ns |
| Portal hypertension | 7 (1%) | 2 (1,9) | Ns | 7 (1%) | 0 | Ns | 2 | 0 | Ns |
| Celiac disease | 26 (4%) | 1 (1) | 0.005 | 24 (4%) | 2 (4%) | Ns | 1 | 0 | Ns |
| Barrett’s esophagus | 25 (4%) | 9 (8.3) | 0.032 | 22 (4%) | 3 (4%) | Ns | 9 | 0 | 0.057 |
| Submucosal lesions | 17 (2%) | 0 | Ns | 15 (2%) | 2 (4%) | Ns | 0 | 0 | - |
| Esophageal stenosis | 5 (0.7%) | 3 * (2.9) | 0.075 | 4 (6%) | 1 (2%) | Ns | 3 | 0 | Ns |
| Benign duodenal stenosis | 3 (0.4%) | 0 | Ns | 3 (0.4%) | 0 | Ns | 0 | 0 | - |
| Esophageal cancer | 7 (1%) | 1 (1) | Ns | 7 (1%) | 0 | Ns | 1 | 0 | Ns |
| Gastric cancer | 17 (2%) | 1 (1) | Ns | 17 (3%) | 0 | Ns | 1 | 0 | Ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceroni, L.; Lodato, F.; Tubertini, P.; Marasco, G.; Gazzola, A.; Biselli, M.; Fabbri, C.; Buonfiglioli, F.; Ferrara, F.; Schiumerini, R.; et al. The Gastropack Access System as a Model to Access Gastroenterology Services for Gastroscopy Appropriateness in Patients with Upper Gastrointestinal Symptoms: A Comparison with the Open Access System. J. Clin. Med. 2023, 12, 3343. https://doi.org/10.3390/jcm12093343
Ceroni L, Lodato F, Tubertini P, Marasco G, Gazzola A, Biselli M, Fabbri C, Buonfiglioli F, Ferrara F, Schiumerini R, et al. The Gastropack Access System as a Model to Access Gastroenterology Services for Gastroscopy Appropriateness in Patients with Upper Gastrointestinal Symptoms: A Comparison with the Open Access System. Journal of Clinical Medicine. 2023; 12(9):3343. https://doi.org/10.3390/jcm12093343
Chicago/Turabian StyleCeroni, Liza, Francesca Lodato, Paolo Tubertini, Giovanni Marasco, Alessia Gazzola, Maurizio Biselli, Cristiano Fabbri, Federica Buonfiglioli, Francesco Ferrara, Ramona Schiumerini, and et al. 2023. "The Gastropack Access System as a Model to Access Gastroenterology Services for Gastroscopy Appropriateness in Patients with Upper Gastrointestinal Symptoms: A Comparison with the Open Access System" Journal of Clinical Medicine 12, no. 9: 3343. https://doi.org/10.3390/jcm12093343