Diagnosis and Management of Malnutrition in Patients with Heart Failure
Abstract
:1. Introduction
1.1. Definitions and Classification
1.2. Prognosis and Consequences of Malnutrition in HF
2. Causes and Pathophysiological Mechanisms of Malnutrition in HF
2.1. Hemodynamic Alterations
2.2. Neurohormonal and Adrenergic Activation and Inflammation
2.3. Hormonal Imbalance
2.4. Protein Degradation
2.5. Transforming Growth Factor Beta Family
3. Nutritional Needs of Patients with HF
3.1. Caloric Requirements
3.2. Protein Requirements
3.3. Carbohydrates (CHO) and Fats
3.4. Fluids and Sodium
3.5. Other Micronutrients
4. Nutritional Particularities in Some Subpopulations of Patients with HF
4.1. Older Patients
4.2. Diabetes Mellitus
4.3. Cardiorenal Syndrome (CRS)
4.4. Anticoagulated Patients
5. Diagnosis of Malnutrition
5.1. Nutritional Screening
5.2. Diagnosis of Malnutrition
5.2.1. Biochemical Markers
5.2.2. Anthropometry
5.2.3. Body Composition
5.3. Screening and Evaluation of Sarcopenia
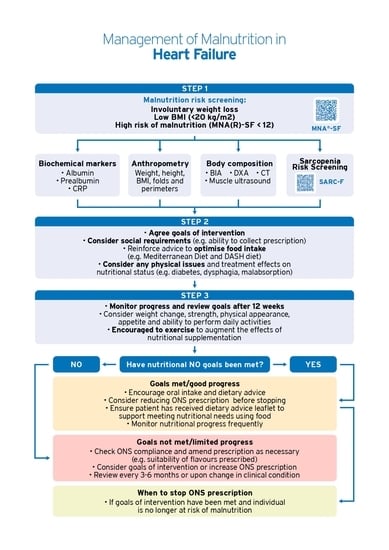
6. Prevention and Nutritional Treatment
Artificial Nutritional Support
7. Drug Treatment and Interactions
| Disorder | Drugs | Monitoring | Treatment |
|---|---|---|---|
| Zinc deficiency | Loop diuretics Thiazide diuretics ACEI/ARA-II | Not recommended as routine [102] Assess whether dysgeusia and treatment with ACEI/ARA-II [96,103] | No routine supplementation [102] Assess whether dysgeusia and treatment with ACEI/ARA-II [96,103] |
| Thiamine deficiency | Loop diuretics | Not recommended as routine [102] Assess whether alcoholic cardiomyopathy [103] | No routine supplementation [102] Assess for alcoholic cardiomyopathy [103] |
| Hypokalemia | Loop diuretics Thiazide diuretics | Every 3–6 months if levels are stable [103] Every 2–4 weeks if the dose start/change: Loop diuretics/thiazides, K supplements (until stability) [103] | Supplementation if there is a deficiency or tendency to hypoK or high doses of diuretic [103] Form of supplementing [103] Potassium-rich diet Pharmacological supplements Optimize ACEI/ARA-II/ARNI, ARM if indicated |
| Hyperkalemia | ACEI/ARA-II/ARNI ARM | Every 3–6 months if levels are stable [103,104] Every 2–4 weeks if the dose start/change: IECA/ARA-II/ARNI, ARM, potassium supplements (until stable) [9] | Low potassium diet [103] Potassium binders [103,105] Caution with ACEI/ARA-II/ARNI/ARM if kidney failure or K > 5 mEq/L [102] |
| Hypomagnesemia | Loop diuretics | Every 3–6 months if levels are stable [99,103] Every week if the dose start/change (until stability) [99,103] | Supplementation if there is a deficiency or tendency to hypoMg and high doses of diuretic [99,103] Form of supplementing [99,103] Mg-rich diet Pharmacological supplements |
| Vitamin B12 deficiency | PPI Metformin | Recommended as routine if metformin [96,101] | Supplementation if deficiency [96] |
| Iron deficiency | PPI | Recommended in all patients with HF [103] | If there is iron deficiency (Ferritin <100 µg/L, or 100–300 µg/L + TSI <20%) with/without anemia, give intravenous iron (oral iron not effective) [103] |
| Coenzyme Q10 deficiency | Statins | Not recommended as routine | Supplementation is not recommended, although there are trials that suggest that it could improve mortality and hospitalizations due to HF [1] |
8. Physical and Functional Rehabilitation
9. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| CV | cardiovascular |
| HF | heart failure |
| LVEF | left ventricular ejection fraction |
| MNA | Mini Nutritional Assessment |
| RAAS | renin-angiotensin-aldosterone system |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic Heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card Fail 2019, 25, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.-M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA®. Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN practical guideline: Clinical nutrition and hydration in geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of malnutrition in older adults: A multinational perspective using the mini nutritional assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Zhang, J.; Clark, A.L. Malnutrition, congestion and mortality in ambulatory patients with Heart failure. Heart 2019, 105, 297–306. [Google Scholar] [CrossRef]
- Wawrzeńczyk, A.; Anaszewicz, M.; Wawrzeńczyk, A.; Budzyński, J. Clinical significance of nutritional status in patients with chronic Heart failure-a systematic review. Heart Fail Rev. 2019, 24, 671–700. [Google Scholar] [CrossRef]
- von Haehling, S.; Lainscak, M.; Springer, J.; Anker, S.D. Cardiac cachexia: A systematic overview. Pharmacol. Ther. 2009, 121, 227–252. [Google Scholar] [CrossRef]
- Sager, R.; Güsewell, S.; Rühli, F.; Bender, N.; Staub, K. Obesity and the risk of Heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar]
- Sánchez, M.A.; Rodríguez, J.L.L.; Freire, R.B.; Colet, J.C.; Leiro, M.G.C.; Vílchez, F.G.; Lorite, N.M.; Cubero, J.S.; Mateas, F.R.; Somoza, F.J.E.; et al. Classification and Quality Standards of Heart Failure Units: Scientific Consensus of the Spanish Society of Cardiology. Rev. Esp. Cardiol. 2016, 69, 940–950. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-Peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.J. Wasting as independent risk factor for mortality in chronic Heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age. Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Saitoh, M.; Valentova, M.; Sandek, A.; Evertz, R.; Ebner, N.; Loncar, G.; Springer, J.; Doehner, W.; Lainscak, M.; et al. Comparison of sarcopenia and cachexia in men with chronic Heart failure: Results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur. J. Heart Fail 2018, 20, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, D.L.; Bohmke, N.; Billingsley, H.E.; Carbone, S. Sarcopenic Obesity in Heart Failure With Preserved Ejection Fraction. Front. Endocrinol. 2020, 11, 558271. [Google Scholar] [CrossRef]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Anguita-Sánchez, M.P.; Castillo-Domínguez, J.C.; García-Fuertes, D.; Crespin-Crespin, M.; López-Granados, A.; de Lezo, J.S. Impact of malnutrition on long-term mortality in hospitalized patients with Heart failure. Rev. Esp. Cardiol. 2011, 64, 752–758. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure: A Comparison With Body Mass Index. JACC Heart Fail 2018, 6, 476–486. [Google Scholar] [CrossRef]
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic Heart failure. ESC Heart Fail 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Packer, M. The neurohormonal hypothesis: A theory to explain the mechanism of disease progression in Heart failure. J. Am. Coll. Cardiol. 1992, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Josiak, K.; Jankowska, E.A.; Piepoli, M.F.; Banasiak, W.; Ponikowski, P. Skeletal myopathy in patients with chronic Heart failure: Significance of anabolic-androgenic hormones. J. Cachexia Sarcopenia Muscle 2014, 5, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Jafry, S.; Jeejeebhoy, K.; Nagpal, A.D.; Pisani, B.; Agarwala, R. Malnutrition and Cachexia in Heart Failure. JPEN J. Parenter. Enteral. Nutr. 2016, 40, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Battin, D.L.; Ali, S.; Shahbaz, A.U.; Munir, A.; Davis, R.C., Jr.; Newman, K.P.; Weber, K.T.; Massie, J.D. Hypoalbuminemia and lymphocytopenia in patients with decompensated biventricular failure. Am. J. Med. Sci. 2010, 339, 31–35. [Google Scholar] [CrossRef]
- Hesse, B.; Parving, H.H.; Lund Jacobsen, H.; Noer, I. Transcapillary escape rate of albumin and right atrial pressure in chronic congestive heart failure before and after treatment. Circ. Res. 1976, 39, 358–362. [Google Scholar] [CrossRef]
- Wrigley, B.J.; Lip, G.Y.H.; Shantsila, E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur. J. Heart Fail 2011, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S. Sympathetic nervous system activation in human heart failure: Clinical implications of an updated model. J. Am. Coll. Cardiol. 2009, 54, 375–385. [Google Scholar] [CrossRef]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef]
- Hryniewicz, K.; Androne, A.S.; Hudaihed, A.; Katz, S.D. Partial reversal of cachexia by beta-adrenergic receptor blocker therapy in patients with chronic Heart failure. J. Card Fail 2003, 9, 464–468. [Google Scholar] [CrossRef]
- Anker, S.D.; Chua, T.P.; Ponikowski, P.; Harrington, D.; Swan, J.W.; Kox, W.J.; Poole-Wilson, P.A.; Coats, A.S. Hormonal changes and catabolic/anabolic imbalance in chronic Heart failure and their importance for cardiac cachexia. Circulation 1997, 96, 526–534. [Google Scholar] [CrossRef]
- Pocock, S.J.; McMurray, J.J.; Dobson, J.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Östergren, J.; Pfeffer, M.A.; Solomon, S.D.; Anker, S.D.; et al. Weight loss and mortality risk in patients with chronic Heart failure in the candesartan in Heart failure: Assessment of reduction in mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Clark, A.L. Catabolism in chronic Heart failure. Eur. Heart J. 2000, 21, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Ballmer, P.E. Causes and mechanisms of hypoalbuminaemia. Clin. Nutr. 2001, 20, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in Heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- Lund, L.H.; Williams, J.J.; Freda, P.; Lamanca, J.J.; Lejemtel, T.H.; Mancini, D.M. Ghrelin resistance occurs in severe Heart failure and resolves after Heart transplantation. Eur. J. Heart Fail 2009, 11, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.D.; Heineke, J. Myostatin from the Heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1973–H1982. [Google Scholar] [CrossRef]
- Sharma, A.; Stevens, S.R.; Lucas, J.; Fiuzat, M.; Adams, K.F.; Whellan, D.J.; Donahue, M.P.; Kitzman, D.W.; Piña, I.L.; Zannad, F.; et al. Utility of Growth Differentiation Factor-15, A Marker of Oxidative Stress and Inflammation, in Chronic Heart Failure: Insights From the HF-ACTION Study. JACC Heart Fail 2017, 5, 724–734. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Sciatti, E.; Lombardi, C.; Ravera, A.; Vizzardi, E.; Bonadei, I.; Carubelli, V.; Gorga, E.; Metra, M. Nutritional deficiency in patients with Heart failure. Nutrients 2016, 8, 442. [Google Scholar] [CrossRef]
- Mijan de la Torre, A.; de Mateo Silleras, B.; AM, P.G. Nutrición e insuficiencia cardiaca. In Nutricion e Insuficiencia Cardiaca En: Ángel Gil Tratado de Nutrición Tomo 5: Nutrición y Enfermedad, 3rd ed.; Panamericana: Madrid, Spain, 2017; pp. 705–728. [Google Scholar]
- Kuehneman, T.; Gregory, M.; de Waal, D.; Davidson, P.; Frickel, R.; King, C.; Gradwell, E.; Handu, D. Academy of Nutrition and Dietetics Evidence-Based Practice Guideline for the Management of Heart Failure in Adults. J. Acad. Nutr. Diet. 2018, 118, 2331–2345. [Google Scholar] [CrossRef]
- Olveira, G.; González Romero, S. Nutrición en el adulto. In Tratado de Nutrición En: Gil Hernández A (Dir), 2nd ed.; Médica Panamericana: Madrid, Spain, 2010; pp. 289–318. [Google Scholar]
- A Ezekowitz, J.; Colin-Ramirez, E.; Ross, H.; Escobedo, J.; Macdonald, P.; Troughton, R.; Saldarriaga, C.; Alemayehu, W.; A McAlister, F.; Arcand, J.; et al. Reduction of dietary sodium to less than 100 mmol in Heart failure (SODIUM-HF): An international, open-label, randomised, controlled trial. Lancet 2022, 399, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.L.; Jeejeebhoy, K.N.; Sole, M.J. The management of conditioned nutritional requirements in Heart failure. Heart Fail Rev. 2006, 11, 75–82. [Google Scholar] [CrossRef]
- Lennie, T.A.; Andreae, C.; Rayens, M.K.; Song, E.K.; Dunbar, S.B.; Pressler, S.J.; Heo, S.; Kim, J.; Moser, D.K. Micronutrient Deficiency Independently Predicts Time to Event in Patients With Heart Failure. J. Am. Heart Assoc. 2018, 7, e007251. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pombo, A.; Rodríguez-Carnero, G.; Castro, A.I.; Cantón-Blanco, A.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B.; Martínez-Olmos, M.A. Relevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic Heart failure. Clin. Nutr. 2021, 40, 5141–5155. [Google Scholar] [CrossRef] [PubMed]
- McKeag, N.A.; McKinley, M.C.; Harbinson, M.T.; Noad, R.L.; Dixon, L.H.; McGinty, A.; Neville, C.E.; Woodside, J.V.; McKeown, P.P. The effect of multiple micronutrient supplementation on left ventricular ejection fraction in patients with chronic stable Heart failure: A randomized, placebo-controlled trial. JACC Heart Fail 2014, 2, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kassis, N.; Hariri, E.H.; Karrthik, A.K.; Ahuja, K.R.; Layoun, H.; Saad, A.M.; Gad, M.M.; Kaur, M.; Bazarbashi, N.; Griffin, B.P.; et al. Supplemental calcium and vitamin D and long-term mortality in aortic stenosis. Heart 2022, 108, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mehta, R.; Al-Ani, M.; Hill, J.A.; Winchester, D.E. Determining the Role of Thiamine Deficiency in Systolic Heart Failure: A Meta-Analysis and Systematic Review. J. Card Fail 2015, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic Heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar]
- Nodari, S.; Triggiani, M.; Campia, U.; Manerba, A.; Milesi, G.; Cesana, B.M.; Gheorghiade, M.; Cas, L.D. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 870–879. [Google Scholar] [CrossRef]
- Kojuri, J.; Ostovan, M.; Rezaian, G.R.; Dialameh, P.A.; Zamiri, N.; Sharifkazemi, M.; Jannati, M. Effect of omega-3 on brain natriuretic peptide and echocardiographic findings in Heart failure: Double-blind placebo-controlled randomized trial. J. Cardiovasc. Dis. Res. 2013, 4, 20–24. [Google Scholar] [CrossRef]
- Masson, S.; Marchioli, R.; Mozaffarian, D.; Bernasconi, R.; Milani, V.; Dragani, L.; Tacconi, M.; Marfisi, R.M.; Borgese, L.; Cirrincione, V.; et al. Plasma n-3 polyunsaturated fatty acids in chronic Heart failure in the GISSI-Heart Failure Trial: Relation with fish intake, circulating biomarkers, and mortality. Am. Heart J. 2013, 165, 208–215.e4. [Google Scholar] [CrossRef] [PubMed]
- Keogh, A.; Fenton, S.; Leslie, C.; Aboyoun, C.; Macdonald, P.; Zhao, Y.C.; Bailey, M.; Rosenfeldt, F. Randomised double-blind, placebo-controlled trial of coenzyme, Q.; therapy in class II and III systolic Heart failure. Heart Lung. Circ. 2003, 12, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P. The effect of coenzyme Q10 on morbidity and mortality in chronic Heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail 2014, 2, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Fotino, A.D.; Thompson-Paul, A.M.; Bazzano, L.A. Effect of coenzyme Q10 supplementation on Heart failure: A meta-analysis. Am. J. Clin. Nutr. 2013, 97, 268–275. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Lin, Z.; Li, X.; Kong, X.; Sun, G. Review of nutritional screening and assessment tools and clinical outcomes in Heart failure. Heart Fail Rev. 2016, 21, 549–565. [Google Scholar] [CrossRef]
- Lv, S.; Ru, S. The prevalence of malnutrition and its effects on the all-cause mortality among patients with Heart failure: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259300. [Google Scholar] [CrossRef]
- Imoberdorf, R.; Meier, R.; Krebs, P.; Hangartner, P.J.; Hess, B.; Stäubli, M.; Wegmann, D.; Rühlin, M.; Ballmer, P.E. Prevalence of undernutrition on admission to Swiss hospitals. Clin. Nutr. 2010, 29, 38–41. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- House, A.A.; Wanner, C.; Sarnak, M.J.; Piña, I.L.; McIntyre, C.W.; Komenda, P.; Kasiske, B.L.; Deswal, A.; Defilippi, C.R.; Cleland, J.G.F.; et al. Heart failure in chronic kidney disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 95, 1304–1317. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.I.; González-Ortiz, A.; Espinosa-Cuevas, A.; Avesani, C.M.; Carrero, J.J.; Cuppari, L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol. Dial. Transplant 2021, 36, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, F.; Dopeux, L.; Mulliez, A.; Boirie, Y.; Morand, C.; Gentes, E.; Farigon, N.; Richard, D.; Lebreton, A.; Teissandier, D.; et al. Severe undernutrition increases bleeding risk on vitamin-K antagonists. Clin. Nutr. 2021, 40, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Harada, M.; Motoike, Y.; Koshikawa, M.; Ichikawa, T.; Watanabe, E.; Ozaki, Y. Impact of serum albumin levels on supratherapeutic PT-INR control and bleeding risk in atrial fibrillation patients on warfarin: A prospective cohort study. Int. J. Cardiol. Heart Vasc. 2019, 22, 111–116. [Google Scholar] [CrossRef]
- Violi, F.; Lip, G.Y.H.; Pignatelli, P.; Pastori, D. Interaction Between Dietary Vitamin K Intake and Anticoagulation by Vitamin K Antagonists: Is It Really True?: A Systematic Review. Medicine 2016, 95, e2895. [Google Scholar] [CrossRef]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef]
- Joaquín, C.; Puig, R.; Gastelurrutia, P.; Lupón, J.; de Antonio, M.; Domingo, M.; Moliner, P.; Zamora, E.; Martin, M.; Alonso, N.; et al. Mini nutritional assessment is a better predictor of mortality than subjective global assessment in Heart failure out-patients. Clin. Nutr. 2019, 38, 2740–2746. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Hirose, S.; Matsue, Y.; Kamiya, K.; Kagiyama, N.; Hiki, M.; Dotare, T.; Sunayama, T.; Konishi, M.; Saito, H.; Saito, K.; et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with Heart failure. Clin. Nutr. 2021, 40, 4334–4340. [Google Scholar] [CrossRef]
- Joaquín, C.; Alonso, N.; Lupón, J.; Gastelurrutia, P.; Pérez-Monstesdeoca, A.; Domingo, M.; Zamora, E.; Socias, G.; Ramos, A.; Bayes-Genis, A.; et al. Nutritional Status According to the GLIM Criteria in Patients with Chronic Heart Failure: Association with Prognosis. Nutrients 2022, 14, 2244. [Google Scholar] [CrossRef]
- Castillo-Martinez, L.; Colin-Ramirez, E.; Orea-Tejeda, A.; Islas, D.G.G.; Rodríguez-García, W.; Díaz, C.S.; Rodríguez, A.E.G.; Durán, M.V.; Davies, C.K. Cachexia assessed by bioimpedance vector analysis as a prognostic indicator in chronic stable Heart failure patients. Nutrition 2012, 28, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Heymsfield, S.B. Lean tissue imaging: A new era for nutritional assessment and intervention. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 940–953. [Google Scholar] [CrossRef]
- Fuentes-Abolafio, I.J.; Ricci, M.; Bernal-López, M.R.; Gómez-Huelgas, R.; Cuesta-Vargas, A.I.; Pérez-Belmonte, L.M. Biomarkers and the quadriceps femoris muscle architecture assessed by ultrasound in older adults with Heart failure with preserved ejection fraction: A cross-sectional study. Aging Clin. Exp. Res. 2022, 34, 2493–2504. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Wu, C.; Jones, M.; Kato, T.S.; Dam, T.T.; Givens, R.C.; Templeton, D.L.; Maurer, M.S.; Naka, Y.; Takayama, H.; et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with Heart failure undergoing ventricular assist device placement. J. Card Fail 2014, 20, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Tran, R.H.; Aldemerdash, A.; Chang, P.; Sueta, C.A.; Kaufman, B.; Asafu-Adjei, J.; Vardeny, O.; Daubert, E.; Alburikan, K.A.; Kucharska-Newton, A.M.; et al. Guideline-Directed Medical Therapy and Survival Following Hospitalization in Patients with Heart Failure. Pharmacotherapy 2018, 38, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Martínez-González, M.; Alonso-Gómez, A.; Rekondo, J.; Salas-Salvadó, J.; Corella, D.; Ros, E.; Fitó, M.; Estruch, R.; Lapetra, J.; et al. Mediterranean diet and risk of Heart failure: Results from the PREDIMED randomized controlled trial. Eur. J. Heart Fail 2017, 19, 1179–1185. [Google Scholar] [CrossRef]
- Miró, Ò.; Estruch, R.; Martín-Sánchez, F.J.; Gil, V.; Jacob, J.; Herrero-Puente, P.; Mateo, S.H.; Aguirre, A.; Andueza, J.A.; Llorens, P. Adherence to Mediterranean Diet and All-Cause Mortality After an Episode of Acute Heart Failure: Results of the MEDIT-AHF Study. JACC Heart Fail 2018, 6, 52–62. [Google Scholar] [CrossRef]
- Lara, K.M.; Levitan, E.B.; Gutierrez, O.M.; Shikany, J.M.; Safford, M.M.; Judd, S.E.; Rosenson, R.S. Dietary Patterns and Incident Heart Failure in US Adults Without Known Coronary Disease. J. Am. Coll. Cardiol. 2019, 73, 2036–2045. [Google Scholar] [CrossRef]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Consistency with the DASH diet and incidence of Heart failure. Arch. Intern. Med. 2009, 169, 851–857. [Google Scholar] [CrossRef]
- Hummel, S.L.; Karmally, W.; Gillespie, B.W.; Helmke, S.; Teruya, S.; Wells, J.; Trumble, E.; Jimenez, O.; Marolt, C.; Wessler, J.D.; et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ. Heart Fail 2018, 11, e004886. [Google Scholar] [CrossRef]
- Tektonidis, T.G.; Åkesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. Adherence to a Mediterranean diet is associated with reduced risk of Heart failure in men. Eur. J. Heart Fail 2016, 18, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tektonidis, T.G.; Åkesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. A Mediterranean diet and risk of myocardial infarction, Heart failure and stroke: A population-based cohort study. Atherosclerosis 2015, 243, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Horwich, T.B.; Fonarow, G.C.; Hamilton, M.A.; MacLellan, W.R.; Woo, M.A.; Tillisch, J.H. The relationship between obesity and mortality in patients with Heart failure. J. Am. Coll. Cardiol. 2001, 38, 789–795. [Google Scholar] [CrossRef]
- Frías, L.; Cuerda, C. Nutrición enteral; indicaciones, sondas y materiales. Nutr. Hosp. 2014, 29 (Suppl. S3), 5–20. [Google Scholar]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Jiménez, F.J.; Cervera Montes, M.; Blesa Malpica, A.L. Guidelines for specialized nutritional and metabolic support in the critically-ill patient. Update. Consensus of the Spanish Society of Intensive Care Medicine and Coronary Units-Spanish Society of Parenteral and Enteral Nutrition (SEMICYUC-SENPE): Card. Med. Intensiva. 2011, 35 (Suppl. S1), 81–85. [Google Scholar] [CrossRef]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Castillo-Domínguez, J.C.; Moreno-Conde, M.; Ibáñez, M.C.L.; Expósito, R.A.; Ortega, E.R.; Anguita-Sánchez, M.P.; Villar-Ráez, A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch. Med. Res. 2016, 47, 535–540. [Google Scholar] [CrossRef]
- Habaybeh, D.; de Moraes, M.B.; Slee, A.; Avgerinou, C. Nutritional interventions for Heart failure patients who are malnourished or at risk of malnutrition or cachexia: A systematic review and meta-analysis. Heart Fail Rev. 2021, 26, 1103–1118. [Google Scholar] [CrossRef]
- Hersberger, L.; Dietz, A.; Bürgler, H.; Bargetzi, A.; Bargetzi, L.; Kägi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; Pavlicek, V.; et al. Individualized Nutritional Support for Hospitalized Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 2307–2319. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Hummel, S.L.; Carbone, S. The role of diet and nutrition in Heart failure: A state-of-the-art narrative review. Prog. Cardiovasc. Dis. 2020, 63, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Cohen, L.P.; Gallagher, D.; Karmally, W.; Maddox, T.; Hummel, S.L.; Carbone, S.; Maurer, M.S. Nutrition Assessment and Dietary Interventions in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Sulo, S.; Walzer, S.; Krenberger, S.; Stagna, Z.; Gomes, F.; Mueller, B.; Brunton, C. Economic Evaluation of Individualized Nutritional Support for Hospitalized Patients with Chronic Heart Failure. Nutrients 2022, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Little, M.O. Updates in nutrition and polypharmacy. Curr. Opin Clin. Nutr. Metab. Care. 2018, 21, 4–9. [Google Scholar] [CrossRef]
- Zenuk, C.; Healey, J.; Donnelly, J.; Vaillancourt, R.; Almalki, Y.; Smith, S. Thiamine deficiency in congestive Heart failure patients receiving long term furosemide therapy. Can. J. Clin. Pharmacol. 2003, 10, 184–188. [Google Scholar]
- Ahmed, A.; Zannad, F.; E Love, T.; Tallaj, J.; Gheorghiade, M.; Ekundayo, O.J.; Pitt, B. A propensity-matched study of the association of low serum potassium levels and mortality in chronic Heart failure. Eur. Heart J. 2007, 28, 1334–1343. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Salinas, G.A.; de Juan Bagudá, J.; Fernández-Fresnedo, G.; Magaña, J.G.; Iniesta, Á.M.; Rivera-Juárez, A.; Marcos, M.C. Fisiopatología, diagnóstico y tratamiento de la hipomagnesemia en pacientes con insuficiencia cardiaca. REC CardioClinics 2021, 56, 299–308. [Google Scholar] [CrossRef]
- McColl, K.E.L. Effect of proton pump inhibitors on vitamins and iron. Am. J. Gastroenterol. 2009, 104, S5–S9. [Google Scholar]
- Fenton, R.; Brook-Barclay, L.; Delaney, C.L.; Spark, J.I.; Miller, M.D. Do Medications Commonly Prescribed to Patients with Peripheral Arterial Disease Have an Effect on Nutritional Status? A Review of the Literature. Ann. Vasc. Surg. 2016, 32, 145–175. [Google Scholar] [CrossRef]
- Edelmann, F.; Gelbrich, G.; Düngen, H.-D.; Fröhling, S.; Wachter, R.; Stahrenberg, R.; Binder, L.; Töpper, A.; Lashki, D.J.; Schwarz, S.; et al. Exercise training improves exercise capacity and diastolic function in patients with Heart failure with preserved ejection fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J. Am. Coll. Cardiol. 2011, 58, 1780–1791. [Google Scholar] [CrossRef]
- Ismail, H.; McFarlane, J.R.; Nojoumian, A.H.; Dieberg, G.; Smart, N.A. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with Heart failure: A systematic review and meta-analysis. JACC Heart Fail 2013, 1, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Walker, S.; Smart, N.A.; Piepoli, M.F.; Warren, F.C.; Ciani, O.; O’Connor, C.; Whellan, D.; Keteyian, S.J.; Coats, A.; et al. Impact of exercise-based cardiac rehabilitation in patients with Heart failure (ExTraMATCH II) on mortality and hospitalisation: An individual patient data meta-analysis of randomised trials. Eur. J. Heart Fail 2018, 20, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Walker, S.; Smart, N.A.; Piepoli, M.F.; Warren, F.C.; Ciani, O.; Whellan, D.; O’Connor, C.; Keteyian, S.J.; Coats, A.; et al. Impact of Exercise Rehabilitation on Exercise Capacity and Quality-of-Life in Heart Failure: Individual Participant Meta-Analysis. J. Am. Coll. Cardiol. 2019, 73, 1430–1443. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Arrarte, V.; Pinilla, J.M.G.; Campuzano, R.; de Pablo, C.; Beltrán, P.; Garcia-Quintana, A.; Almenar, L.; Bover, R.; Ortiz, C.; et al. Consenso de expertos en la asistencia multidisciplinaria y el abordaje integral de la insuficiencia cardiaca. Desde el alta hospitalaria hasta la continuidad asistencial con primaria. Rev. Española. Cardiol. 2020, 20, 3–12. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Conraads, V.; Corrà, U.; Dickstein, K.; Francis, D.P.; Jaarsma, T.; Mcmurray, J.; Pieske, B.; Piotrowicz, E.; Schmid, J.-P.; et al. Exercise training in Heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Heart Fail 2011, 13, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients With Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef]


| Nutritional Needs | Recommendations | Effects | |
|---|---|---|---|
| Caloric needs | 22 kcal/kg (per actual weight) in normally nourished patients 24 kcal/kg per actual weight in malnourished patients | An adequate intake has been shown to improve quality of life. A reduced or excessive intake can cause decompensated HF | |
| Protein needs | 1.1 to 1.4 g/kg/day using actual weight | It promotes muscle synthesis, improving physical capacity and muscle mass | |
| Liquids | 1.5 to 2 L per day * (taking all daily fluid intake into account) | Adjust according to acute or chronic phase or hyponatremia | |
| Sodium | 2–3 g/day | Adjust according to acute or chronic phase or hyponatremia | |
| Other electrolytes (potassium, magnesium, calcium) | Only if there is a deficiency (with oral supplementation and/or increased intake of electrolyte-rich foods) | Frequent deficiency due to the use of diuretics. Severe hypocalcemia can cause cardiac dysfunction. | |
| Fat-soluble vitamins (A,D,E,K) and water-soluble vitamins (B6,B12) | Supplement if deficiency of “general recommended daily doses”. | Less frequent deficiencies, except for vitamin D deficiency. | |
| Iron | If there is a deficiency (ferritin <100 ng/mL or 100–300 ng/mL with TSI < 20%). Administer iv | Deficiency can worsen HF functional status and quality of life. | |
| Thiamine | If there is a deficiency, correct according to general recommendations. Low levels can worsen heart function | Decreased heart function. Severe deficiency leads to reversible cardiomyopathy | |
| Coenzyme Q10 | Its use may be considered. Variable dose (60 to 300 mg/day) | Deficiency associated with poorer cardiac function and biomarkers | |
| PUFA: Omega 3 | It could be considered (dose 1 to 2 mg/day). It may reduce the risk of admission for HF and/or CV death | A deficiency can increase mortality and readmissions in HF | |
| Other micronutrients | Zinc, selenium, folate | Only if there is a deficiency (with oral supplementation and/or increased intake of electrolyte-rich foods) | Deficiency related to increased renal excretion. Severe selenium deficiency leads to reversible cardiomyopathy |
| 1 Phenotypic Criterion + 1 Etiological Criterion = Diagnosis of Malnutrition | |||||
|---|---|---|---|---|---|
| Phenotypic Criteria | Etiological Criteria | ||||
| Weight loss (%) | Low BMI (kg/m2) | Reduced muscle mass | Reduced dietary intake or absorption | Inflammation | |
| Moderate malnutrition | 5–10% in the last 6 months or 10–20% in >6 months | <20 in <70 years or <22 in ≥70 years | Mild to moderate deficiency * | ≤50% of energy requirements or any reduction over >2 weeks or any condition that affects food absorption | Related to an acute illness or injury Related to chronic disease |
| Severe malnutrition | >10% in the last 6 months or >20% in more than 6 months | <18.5 in <70 years or <20 in ≥70 years | Severe deficiency * | ||
| Advantages | Disadvantages | |
|---|---|---|
| Anthropometry | Economical Accessible | Subclinical volume overload and changes in hydration status due to worsening of HF or in response to therapy complicate assessment. |
| Bioimpedance | Fast Does not involve radiation Inexpensive Easy to use | Estimation of body composition based on mathematical calculations. Limited use in patients with abnormal blood volume Contraindicated in patients with implantable cardiac devices |
| DXA | Easy to use Lower cost than CT or MRI. It permits the assessment of the 3 compartments: muscle, adipose, and bone. | Radiation exposure (albeit minimal) Major changes in hydration status (>5%) may overestimate fat-free mass. |
| CT | It permits the analysis of muscle mass, fat mass, and the distribution thereof (subcutaneous, visceral, and intramuscular) | High cost Radiation exposure |
| Muscle ultrasound | Simple Low cost Good correlation with the data obtained by MRI. | Cut-off points for low muscle mass are not universally defined. |
| Weight Loss and Early Satiety |
| Low-volume meals divided into 5–6 meals |
| Low-volume and calorie- and protein-enriched dishes |
| Liberalized diet according to patient preferences |
| Drink fluids between meals |
| Foods that are easy to chew and swallow |
| Difficulty Chewing |
| Cook food thoroughly and avoid tougher foods |
| Foods that are easy to chew and swallow |
| Difficulty Swallowing |
| Homogeneous diet with cream texture |
| Liquids with thickeners |
| Nausea or Dyspepsia |
| Easily-digestible diet |
| Eliminate foods that cause symptoms |
| Altered Intestinal Transit |
| Increase dietary fiber |
| Protein Malnutrition | Protein-Energy Malnutrition | Malnutrition with Altered Bowel Habits | Protein Malnutrition with Obesity | |||
|---|---|---|---|---|---|---|
| General | Complete diet Normocaloric Hyperproteic With fiber | Complete diet Hypercaloric Hyperproteic With fiber | Complete diet Normocaloric Hyperproteic With fiber, without fiber, or with soluble fiber | Protein module | ||
| Specific | Metabolic Disorders - Diabetes - Insulin resistance | Complete diet Normocaloric Hyperproteic With fiber | Complete diet Hypercaloric Hyperproteic With fiber | Complete diet Normocaloric Hyperproteic Without fiber, or with soluble fiber | Protein module | |
| Patient with Kidney Disease | Pre-dialysis | Complete diet Hypercaloric Hypoproteic With fiber | Complete diet Hypercaloric Hypoproteic With fiber | Complete diet Normocaloric Hypoproteic Without fiber | ||
| Dialysis | Complete diet Normocaloric Hyperproteic With fiber | Complete diet Hypercaloric Hyperproteic With fiber | Complete diet Normocaloric Hyperproteic Without fiber | Protein module | ||
| Dysphagia | Complete diet Modified-texture Hypercaloric and Hyperproteic | Complete diet Modified-texture Hypercaloric and Hyperproteic | Complete diet Modified-texture Hypercaloric and Hyperproteic | Diluted protein module and thickened to modified texture | ||
| Screening Criteria |
| Patients with HF regardless of LVEF, stable, NYHA functional class I–IV, with optimal medical treatment (including stable patients for whom treatment optimization is being completed), without contraindications or limitations for physical exercise. |
| Exclusion Criteria |
| Contraindication for physical exercise: Severe left ventricular outflow tract obstruction: severe aortic stenosis, severe hypertrophic obstructive cardiomyopathy. Advanced atrioventricular block. |
| Temporary contraindications. |
| Uncontrolled diabetes mellitus. Uncontrolled arterial hypertension. Uncontrolled arrhythmias. Myocarditis or pericarditis. Systemic infection. If <48 h have elapsed since an acute coronary syndrome. Intracardiac thrombus |
| Other |
| Partial or total dependency with scant family support or any physical, mental, or social disability preventing them from committing to carrying out the program. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban-Fernández, A.; Villar-Taibo, R.; Alejo, M.; Arroyo, D.; Bonilla Palomas, J.L.; Cachero, M.; Joaquin, C.; Méndez Bailón, M.; Pérez-Rivera, J.Á.; Romero-Vigara, J.C.; et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. J. Clin. Med. 2023, 12, 3320. https://doi.org/10.3390/jcm12093320
Esteban-Fernández A, Villar-Taibo R, Alejo M, Arroyo D, Bonilla Palomas JL, Cachero M, Joaquin C, Méndez Bailón M, Pérez-Rivera JÁ, Romero-Vigara JC, et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. Journal of Clinical Medicine. 2023; 12(9):3320. https://doi.org/10.3390/jcm12093320
Chicago/Turabian StyleEsteban-Fernández, Alberto, Rocío Villar-Taibo, Mirian Alejo, David Arroyo, Juan Luis Bonilla Palomas, Montserrat Cachero, Clara Joaquin, Manuel Méndez Bailón, José Ángel Pérez-Rivera, Juan Carlos Romero-Vigara, and et al. 2023. "Diagnosis and Management of Malnutrition in Patients with Heart Failure" Journal of Clinical Medicine 12, no. 9: 3320. https://doi.org/10.3390/jcm12093320