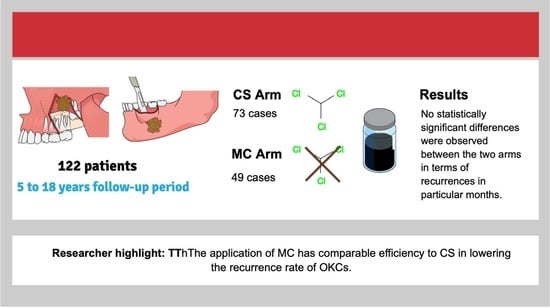
Modified Carnoy’s Versus Carnoy’s Solution in the Management of Odontogenic Keratocysts—A Single Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
- -
- Presence of histopathologically diagnosed parakeratinized OKC in maxilla or mandible
- -
- Unilocular lesions
- -
- Nonsyndromic OKC
- -
- Capacity for giving consent
- -
- Signed informed consent from the patient or guardian
- -
- Patients who underwent surgeries (enucleation, curettage, peripheral osteotomy and subsequent CS or MC application) and completed the follow-up by October 2022
- -
- Follow-up period of at least 5 years.
- -
- Cases with incomplete clinical, radiological, or histopathologic data
- -
- Patients with history of previous surgeries and recurrence of OKC
- -
- Cases in which marsupialization was mandatory due to large size, cortical perforation, or proximity of the sinus membrane
- -
- Cases in which segmental resection was performed due to severe bone resorption or pathological fracture
- -
- Patients diagnosed with Gorlin’s syndrome or nevoid basal cell carcinoma syndrome.
2.3. Intervention
2.4. Statistical Analysis
3. Results
Patient Characteristics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emerson, T.G.; Whitlock, R.I.; Jones, J.H. Involvement of soft tissue by odontogenic keratocysts (primordial cysts). Br. J. Oral Surg. 1972, 9, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Worrall, S.F. Recurrent odontogenic keratocyst within the temporalis muscle. Br. J. Oral Maxillofac. Surg. 1992, 30, 59–62. [Google Scholar] [CrossRef]
- Yamamoto, K.; Matsusue, Y.; Kurihara, M.; Takahashi, Y.; Kirita, T. A keratocyst in the buccal mucosa with the features of keratocystic odontogenic tumor. Open Dent. J. 2013, 7, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Stoelinga, P.J.W. The odontogenic keratocyst revisited. Int. J. Oral Maxillofac. Surg. 2022, 51, 1420–1423. [Google Scholar] [CrossRef]
- Stoelinga, P.J. Excision of the overlying, attached mucosa, in conjunction with cyst enucleation and treatment of the bony defect with carnoy solution. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Stoelinga, P.J.W.; da Silva, Y.S. The significance of recurrent odontogenic keratocysts in bone grafts. Int. J. Oral Maxillofac. Surg. 2021, 50, 746–749. [Google Scholar] [CrossRef]
- DeGould, M.D.; Goldberg, J.S. Recurrence of an odontogenic keratocyst in a bone graft. Report of a case. Int. J. Oral Maxillofac. Surg. 1991, 20, 9–11. [Google Scholar] [CrossRef]
- Persson, G. Remarkable recurrence of a keratocyst in a bone-graft. Int. J. Oral Surg. 1973, 2, 69–76. [Google Scholar] [CrossRef]
- Attenborough, N.R. Recurrence of an odontogenic keratocyst in a bone graft: Report of a case. Br. J. Oral Surg. 1974, 12, 33–39. [Google Scholar] [CrossRef]
- Karaca, C.; Dere, K.; Er, N.; Aktas, A.; Tosun, E.; Koseoglu, O.; Usubutun, A. Recurrence rate of odontogenic keratocyst treated by enucleation and peripheral ostectomy: Retrospective case series with up to 12 years of follow-up. Med. Oral Patol. Oral Cir. Bucal. 2018, 23, e443–e448. [Google Scholar] [CrossRef]
- de Castro, M.S.; Caixeta, C.A.; de Carli, M.L.; Júnior, N.V.R.; Miyazawa, M.; Pereira, A.A.C.; Sperandio, F.F.; Hanemann, J.A.C. Conservative surgical treatments for nonsyndromic odontogenic keratocysts: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2089–2101. [Google Scholar] [CrossRef]
- Kaczmarzyk, T.; Mojsa, I.; Stypulkowska, J. A systematic review of the recurrence rate for keratocystic odontogenic tumour in relation to treatment modalities. Int. J. Oral Maxillofac. Surg. 2012, 41, 756–767. [Google Scholar] [CrossRef]
- Cutler, E.C.; Zollinger, R. The use of sclerosing solutions in the treatment of cysts and fistulae. Am. J. Surg. 1933, 19, 411–418. [Google Scholar] [CrossRef]
- Voorsmit, R.A.; Stoelinga, P.J.; van Haelst, U.J. The management of keratocysts. J. Maxillofac. Surg. 1981, 9, 228–236. [Google Scholar] [CrossRef]
- Lal, B.; Kumar, R.D.; Alagarsamy, R.; Sundaram, D.S.; Bhutia, O.; Roychoudhury, A. Role of Carnoy’s solution as treatment adjunct in jaw lesions other than odontogenic keratocyst: A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, 729–741. [Google Scholar] [CrossRef]
- Ecker, J.; Horst, R.T.; Koslovsky, D. Current Role of Carnoy’s Solution in Treating Keratocystic Odontogenic Tumors. J. Oral Maxillofac. Surg. 2016, 74, 278–282. [Google Scholar] [CrossRef]
- Donnelly, L.A.; Simmons, T.H.; Blitstein, B.J.; Pham, M.H.; Saha, P.T.; Phillips, C.; White, R.P.; Blakey, G.H. Modified Carnoy’s Compared to Carnoy’s Solution Is Equally Effective in Preventing Recurrence of Odontogenic Keratocysts. J. Oral Maxillofac. Surg. 2021, 79, 1874–1881. [Google Scholar] [CrossRef]
- Dashow, J.E.; McHugh, J.B.; Braun, T.M.; Edwards, S.P.; Helman, J.I.; Ward, B.B. Significantly Decreased Recurrence Rates in Keratocystic Odontogenic Tumor With Simple Enucleation and Curettage Using Carnoy’s Versus Modified Carnoy’s Solution. J. Oral Maxillofac. Surg. 2015, 73, 2132–2135. [Google Scholar] [CrossRef]
- Levorová, J.; Machoň, V.; Grill, P.; Hirjak, D.; Foltán, R. Keratocystic Odontogenic Tumour with Extraosseal Spread: Evaluation of the Effect Carnoy’s Solution. Prague Med. Rep. 2015, 116, 303–313. [Google Scholar] [CrossRef]
- Morgan, T.A.; Burton, C.C.; Qian, F. A retrospective review of treatment of the odontogenic keratocyst. J. Oral Maxillofac. Surg. 2005, 63, 635–639. [Google Scholar] [CrossRef]
- Caminiti, M.F.; El-Rabbany, M.; Jeon, J.; Bradley, G. 5-Fluorouracil Is Associated With a Decreased Recurrence Risk in Odontogenic Keratocyst Management: A Retrospective Cohort Study. J. Oral Maxillofac. Surg. 2021, 79, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Hellstein, J.; Hopkins, T.; Morgan, T. The History and Mystery of Carnoy Solution: An Assessment of the Need For Chlorofom. Oral Surg. Oral Med. Oral Radiol. 2007, 103, e24. [Google Scholar] [CrossRef]
- Stoelinga, P.J. Long-term follow-up on keratocysts treated according to a defined protocol. Int. J. Oral Maxillofac. Surg. 2001, 30, 14–25. [Google Scholar] [CrossRef] [PubMed]
| Variable | Treatment Group | Statistical Test Results | |||||
|---|---|---|---|---|---|---|---|
| CS | MC | ||||||
| n | % | n | % | ||||
| Sex | Female | 24 | 32.9 | 18 | 36.7 | χ2(1) = 0.19; p = 0.7 | |
| Male | 49 | 67.1 | 31 | 63.3 | |||
| Location | Mandible | − | 17 | 23.3 | 11 | 22.4 | χ2(1) = 0.01; p > 0.99 |
| + | 56 | 76.7 | 38 | 77.6 | |||
| Maxilla | − | 56 | 76.7 | 38 | 77.6 | χ2(1) = 0.01; p > 0.99 | |
| + | 17 | 23.3 | 11 | 22.4 | |||
| Anterior | − | 53 | 72.6 | 36 | 73.5 | χ2(1) = 0.01; p > 0.99 | |
| + | 20 | 27.4 | 13 | 26.5 | |||
| Posterior | − | 20 | 27.4 | 13 | 26.5 | χ2(1) = 0.01; p > 0.99 | |
| + | 53 | 52.6 | 36 | 73.5 | |||
| Clinical presentation at baseline | Asymptomatic | − | 26 | 35.6 | 18 | 36.7 | χ2(1) = 0.02; p > 0.99 |
| + | 47 | 64.4 | 31 | 63.3 | |||
| Edema | − | 47 | 64.4 | 31 | 63.3 | χ2(1) = 0.02; p > 0.99 | |
| + | 26 | 35.6 | 18 | 36.7 | |||
| Pain | − | 47 | 64.4 | 31 | 63.3 | χ2(1) = 0.02; p > 0.99 | |
| + | 26 | 35.6 | 18 | 36.7 | |||
| Pus discharge | − | 55 | 75.3 | 37 | 75.5 | χ2(1) = 0; p > 0.99 | |
| + | 18 | 24.7 | 12 | 24.5 | |||
| Extraction of adjacent teeth | − | 8 | 11 | 1 | 2 | χ2(1) = 3.41; p = 0.08 | |
| + | 65 | 89 | 48 | 98 | |||
| Bone grafting | − | 50 | 68.5 | 43 | 87.8 | χ2(1) = 6; p = 0.02; ø = 0.22 | |
| + | 23 | 31.5 | 6 | 12.2 | |||
| Month | Arm | Statistical Test Results | ||||
|---|---|---|---|---|---|---|
| CS | MC | |||||
| n | % | n | % | |||
| 3 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| 6 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| 12 | − | 73 | 100 | 48 | 98 | χ2(1) = 1.5; p = 0.4 |
| + | 0 | 0 | 1 | 2 | ||
| 24 | − | 72 | 98.6 | 48 | 98 | χ2(1) = 0.08; p > 0.99 |
| + | 1 | 1.4 | 1 | 2 | ||
| 36 | − | 70 | 95.9 | 47 | 95.9 | χ2(1) = 0; p > 0.99 |
| + | 3 | 4.1 | 2 | 4.1 | ||
| 48 | − | 72 | 98.6 | 48 | 98 | χ2(1) = 0.08; p > 0.99 |
| + | 1 | 1.4 | 1 | 2 | ||
| 60 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| 72 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| 84 | − | 72 | 98.6 | 49 | 100 | χ2(1) = 0.68; p > 0.99 |
| + | 1 | 1.4 | 0 | 0 | ||
| 96 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| 108 | − | 73 | 100 | 49 | 100 | - |
| + | 0 | 0 | 0 | 0 | ||
| Total number of recurrences | 6 | 8.2% | 5 | 10.2% | - | |
| Variable | Total Number of Recurrences in the CS Arm | Statistical Test Results | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | ||||||
| n | % | n | % | ||||
| Sex | Female | 22 | 32.8 | 2 | 33.3 | χ2(1) = 0; p > 0.99 | |
| Male | 45 | 67.2 | 4 | 66.7 | |||
| Location | Mandible | − | 16 | 23.9 | 1 | 16.7 | χ2(1) = 0.16; p > 0.99 |
| + | 51 | 76.1 | 5 | 83.3 | |||
| Maxilla | − | 51 | 76.1 | 5 | 83.3 | χ2(1) = 0.16; p > 0.99 | |
| + | 16 | 23.9 | 1 | 16.7 | |||
| Anterior | − | 49 | 73.1 | 4 | 66.7 | χ2(1) = 0.12; p = 0.66 | |
| + | 18 | 26.9 | 2 | 33.3 | |||
| Posterior | − | 18 | 26.9 | 2 | 33.3 | χ2(1) = 0.12; p = 0.66 | |
| + | 49 | 73.1 | 4 | 66.7 | |||
| Clinical presentation at baseline | Asymptomatic | − | 22 | 32.8 | 4 | 66.7 | χ2(1) = 2.75; p = 0.18 |
| + | 45 | 67.2 | 2 | 33.3 | |||
| Edema | − | 45 | 67.2 | 2 | 33.3 | χ2(1) = 2.75; p = 0.18 | |
| + | 22 | 32.8 | 4 | 66.7 | |||
| Pain | − | 45 | 67.2 | 2 | 33.3 | χ2(1) = 2.75; p = 0.18 | |
| + | 22 | 32.8 | 4 | 66.7 | |||
| Pus discharge | − | 52 | 77.6 | 3 | 50 | χ2(1) = 2.26; p = 0.16 | |
| + | 15 | 22.4 | 3 | 50 | |||
| Extraction of adjacent teeth | − | 7 | 10.4 | 1 | 16.7 | χ2(1) = 0.22; p = 0.52 | |
| + | 60 | 89.6 | 5 | 83.3 | |||
| Bone grafting | − | 45 | 67.2 | 5 | 83.3 | χ2(1) = 0.67; p = 0.66 | |
| + | 22 | 32.8 | 1 | 16.7 | |||
| Treatment | CS | − | − | − | − | − | - |
| + | 67 | 100 | 6 | 100 | |||
| MC | − | 67 | 100 | 6 | 100 | - | |
| + | − | − | − | − | |||
| Variable | Total Number of Recurrences in the MC Arm | Statistical Test Results | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | ||||||
| n | % | n | % | ||||
| Sex | Female | 17 | 38.6 | 1 | 20 | χ2(1) = 0.67; p = 0.64 | |
| Male | 27 | 61.4 | 4 | 80 | |||
| Location | Mandible | − | 8 | 18.2 | 3 | 60 | χ2(1) = 4.51; p = 0.03; ø = 0.3 |
| + | 36 | 81.8 | 2 | 40 | |||
| Maxilla | − | 36 | 81.8 | 2 | 40 | χ2(1) = 4.51; p = 0.03; ø = 0.3 | |
| + | 8 | 18.2 | 3 | 60 | |||
| Anterior | − | 33 | 75 | 3 | 60 | χ2(1) = 0.52; p = 0.6 | |
| + | 11 | 25 | 2 | 40 | |||
| Posterior | − | 11 | 25 | 2 | 40 | χ2(1) = 0.52; p = 0.6 | |
| + | 33 | 75 | 3 | 60 | |||
| Clinical presentation at baseline | Asymptomatic | − | 15 | 34.1 | 3 | 60 | χ2(1) = 1.3; p = 0.34 |
| + | 29 | 65.9 | 2 | 40 | |||
| Edema | − | 29 | 65.9 | 2 | 40 | χ2(1) = 1.3; p = 0.34 | |
| + | 15 | 31.4 | 3 | 60 | |||
| Pain | − | 29 | 65.9 | 2 | 40 | χ2(1) = 1.3; p = 0.34 | |
| + | 15 | 31.4 | 3 | 60 | |||
| Pus discharge | − | 35 | 79.5 | 2 | 40 | χ2(1) = 3.8; p = 0.09 | |
| + | 9 | 20.5 | 3 | 60 | |||
| Extraction of adjacent teeth | − | 1 | 2.3 | 0 | 0 | χ2(1) = 0.12; p = 1 | |
| + | 43 | 97.7 | 5 | 100 | |||
| Bone grafting | − | 38 | 86.4 | 5 | 100 | χ2(1) = 0.78; p = 1 | |
| + | 6 | 13.6 | 0 | 0 | |||
| Treatment | CS | − | 24 | 54.5 | 3 | 60 | χ2(1) = 0.05; p = 1 |
| + | 20 | 45.5 | 2 | 40 | |||
| MC | − | 20 | 45.5 | 2 | 40 | χ2(1) = 0.05; p = 1 | |
| + | 24 | 54.5 | 3 | 60 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janas-Naze, A.; Zhang, W.; Szuta, M. Modified Carnoy’s Versus Carnoy’s Solution in the Management of Odontogenic Keratocysts—A Single Center Experience. J. Clin. Med. 2023, 12, 1133. https://doi.org/10.3390/jcm12031133
Janas-Naze A, Zhang W, Szuta M. Modified Carnoy’s Versus Carnoy’s Solution in the Management of Odontogenic Keratocysts—A Single Center Experience. Journal of Clinical Medicine. 2023; 12(3):1133. https://doi.org/10.3390/jcm12031133
Chicago/Turabian StyleJanas-Naze, Anna, Wei Zhang, and Mariusz Szuta. 2023. "Modified Carnoy’s Versus Carnoy’s Solution in the Management of Odontogenic Keratocysts—A Single Center Experience" Journal of Clinical Medicine 12, no. 3: 1133. https://doi.org/10.3390/jcm12031133