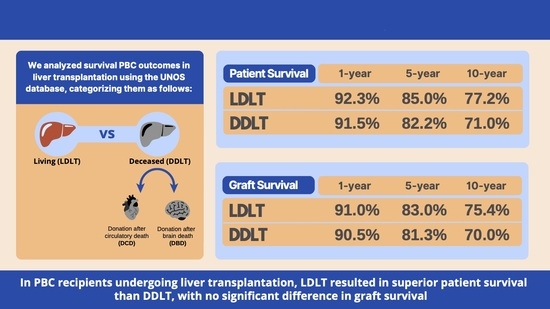
Two Decades of Liver Transplants for Primary Biliary Cholangitis: A Comparative Study of Living Donors vs. Deceased Donor Liver Transplantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Variables
2.3. Outcomes Definitions
2.4. Statistical Analysis
3. Results
3.1. Recipients and Donor Characteristics
3.2. Graft and Patient Survival
3.2.1. Patients Undergoing LDLTs Showed Superior Patient Survival Compared to DDLT Recipients
3.2.2. LDLT Patients Showed Similar Graft Failure Rates Compared to DDLT Recipients
3.3. Risk Factors of Patient Mortality and Graft Failure
3.3.1. Risk Factors for Patient Mortality
3.3.2. Risk Factors for Graft Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barba Bernal, R.; Ferrigno, B.; Medina Morales, E.; Castro, C.M.; Goyes, D.; Trivedi, H.; Patwardhan, V.R.; Bonder, A. Management of primary biliary cholangitis: Current treatment and future perspectives. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2023, 34, 89–100. [Google Scholar] [CrossRef]
- Boonstra, K.; Kunst, A.E.; Stadhouders, P.H. Rising incidence and prevalence of primary biliary cirrhosis: A large population-based study. Liver Int. 2014, 34, e31–e38. [Google Scholar] [CrossRef] [PubMed]
- Shahab, O.; Sayiner, M.; Paik, J.; Felix, S.; Golabi, P.; Younossi, Z.M. Burden of Primary Biliary Cholangitis among Inpatient Population in the United States. Hepatol. Commun. 2019, 3, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef] [PubMed]
- Barba Bernal, R.; Medina-Morales, E.; Goyes, D.; Patwardhan, V.; Bonder, A. Management of Autoimmune Liver Diseases after Liver Transplantation. Transplantology 2021, 2, 162–182. [Google Scholar] [CrossRef]
- Prince, M.I.; Chetwynd, A.; Craig, W.L.; Metcalf, J.V.; James, O.F.W. Asymptomatic primary biliary cirrhosis: Clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004, 53, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Khungar, V.; Goldberg, D.S. Liver Transplantation for Cholestatic Liver Diseases in Adults. Clin. Liver Dis. 2016, 20, 191–203. [Google Scholar] [CrossRef]
- Engel, B.; Taubert, R.; Jaeckel, E.; Manns, M.P. The future of autoimmune liver diseases—Understanding pathogenesis and improving morbidity and mortality. Liver Int. 2020, 40 (Suppl. S1), 149–153. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.A.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014, 147, 1338–1349.e5, quiz e15. [Google Scholar] [CrossRef]
- Harms, M.H.; Lammers, W.J.; Thorburn, D.; Corpechot, C.; Invernizzi, P.; Janssen, H.L.A.; Battezzati, P.M.; Nevens, F.; Lindor, K.D.; Floreani, A.; et al. Major Hepatic Complications in Ursodeoxycholic Acid-Treated Patients with Primary Biliary Cholangitis: Risk Factors and Time Trends in Incidence and Outcome. Am. J. Gastroenterol. 2018, 113, 254–264. [Google Scholar] [CrossRef]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019, 69, 394–419. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Levy, C. Primary Biliary Cholangitis Guidance Update: Implications for Liver Transplantation. Liver Transplant. 2018, 24, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Fang, X.; Kaif, M.; Hasanin, M.; Mcguire, B.M.; Kuo, Y.-F.; Wiesner, R.H. Primary biliary cirrhosis has high wait-list mortality among patients listed for liver transplantation. Transpl. Int. 2017, 30, 454–462. [Google Scholar] [CrossRef]
- Singal, A.K.; Wong, R.J.; Jalan, R.; Asrani, S.; Kuo, Y.-F. Primary biliary cholangitis has the highest waitlist mortality in patients with cirrhosis and acute on chronic liver failure awaiting liver transplant. Clin. Transplant. 2021, 35, e14479. [Google Scholar] [CrossRef] [PubMed]
- Samstein, B.; Smith, A.R.; Freise, C.E.; Zimmerman, M.A.; Baker, T.; Olthoff, K.M.; Fisher, R.A.; Merion, R.M. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings from the A2ALL Cohort Study. Am. J. Transplant. 2016, 16, 594–602. [Google Scholar] [CrossRef]
- Quintini, C.; Hashimoto, K.; Uso, T.D.; Miller, C. Is there an advantage of living over deceased donation in liver transplantation? Transpl. Int. 2013, 26, 11–19. [Google Scholar] [CrossRef]
- Klein, A.S.; Messersmith, E.E.; Ratner, L.E.; Kochik, R.; Baliga, P.K.; Ojo, A.O. Organ donation and utilization in the United States, 1999–2008. Am. J. Transplant. 2010, 10, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, I.A.; Alexopoulos, S.P.; Matsuoka, L.K.; Geevarghese, S.K.; Gorden, L.D.; Karp, S.J.; Perkins, J.D.; Montenovo, M.I. Living vs deceased donor liver transplantation in cholestatic liver disease: An analysis of the OPTN database. Clin. Transplant. 2020, 34, e14031. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Goyes, D.; Barba, R.; Medina-Morales, E.; Saberi, B.; Patwardhan, V.; Bonder, A. Waitlist mortality in patients with autoimmune liver diseases. Ann. Hepatol. 2022, 27, 100742. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Guturu, P.; Hmoud, B.; Kuo, Y.-F.; Salameh, H.; Wiesner, R.H. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation 2013, 95, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Stepanova, M.; De Avila, L.; Golabi, P.; Racila, A.; Younossi, Z.M. Outcomes of Liver Transplant Candidates with Primary Biliary Cholangitis: The Data from the Scientific Registry of Transplant Recipients. Dig. Dis. Sci. 2020, 65, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Safadjou, S.; Chen, R.; Mantry, P.; Sharma, R.; Patil, V.; Maloo, M.; Ryan, C.; Marroquin, C.; Barry, C.; et al. Living Donor and Deceased Donor Liver Transplantation for Autoimmune and Cholestatic Liver Diseases—An Analysis of the UNOS Database. J. Gastrointest. Surg. 2010, 14, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, M.; Liwinski, T.; Adam, R.; Berenguer, M.; Mirza, D.; Malek-Hosseini, S.A.; Heneghan, M.A.; Lodge, P.; Pratschke, J.; Boudjema, K.; et al. Long-term outcome after living donor liver transplantation compared to donation after brain death in autoimmune liver diseases: Experience from the European Liver Transplant Registry. Am. J. Transplant. 2022, 22, 626–633. [Google Scholar] [CrossRef]
- Egawa, H.; Sakisaka, S.; Teramukai, S.; Sakabayashi, S.; Yamamoto, M.; Umeshita, K.; Uemoto, S. Long-Term Outcomes of Living-Donor Liver Transplantation for Primary Biliary Cirrhosis: A Japanese Multicenter Study. Am. J. Transplant. 2016, 16, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.E.; Garcia, R.F.L.; Gunson, B.; Christensen, E.; Neuberger, J.; McMaster, P.; Mirza, D.F. Analysis of marginal donor parameters in liver transplantation for primary biliary cirrhosis. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2004, 2, 183–188. [Google Scholar]
- Sierra, L.; Barba, R.; Ferrigno, B.; Goyes, D.; Diaz, W.; Patwardhan, V.R.; Saberi, B.; Bonder, A. Living-Donor Liver Transplant and Improved Post-Transplant Survival in Patients with Primary Sclerosing Cholangitis. J. Clin. Med. 2023, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]


| Variable | LDLT (n = 464) | DCD (n = 230) | DBD (n = 3237) | p-Value |
|---|---|---|---|---|
| RECIPIENT CHARACTERISTICS | ||||
| Age (years) | 55 (48, 62) | 59 (52, 65) | 57 (50, 64) | <0.001 |
| Gender, n (%) | ||||
| Female | 397 (85.6%) | 186 (80.9%) | 2669 (82.5%) | 0.19 |
| Male | 67 (14.4%) | 44 (19.1%) | 568 (17.5%) | |
| Race, n (%) | ||||
| White | 375 (80.8%) | 174 (75.7%) | 2319 (71.6%) | <0.001 |
| Black | 14 (3.0%) | 18 (7.8%) | 255 (7.9%) | |
| Hispanic | 67 (14.4%) | 29 (12.6%) | 521 (16.1%) | |
| Asian | 3 (0.6%) | 6 (2.6%) | 94 (2.9%) | |
| Other | 5 (1.1%) | 3 (1.3%) | 48 (1.5%) | |
| DM, n (%) | 51 (11.0%) | 36 (15.7%) | 478 (14.8%) | 0.081 |
| Blood type, n (%) | ||||
| O | 218 (47.0%) | 92 (40.0%) | 1498 (46.3%) | 0.004 |
| A | 185 (39.9%) | 87 (37.8%) | 1165 (36.0%) | |
| B | 53 (11.4%) | 41 (17.8%) | 407 (12.6%) | |
| AB | 8 (1.7%) | 10 (4.3%) | 167 (5.2%) | |
| BMI | 25.2 (22.7, 28.9) | 26.7 (23.6, 30.7) | 26.3 (23.2, 30.3) | <0.001 |
| Life support, n (%) | ||||
| No | 464 (100.0%) | 227 (98.7%) | 3198 (98.8%) | 0.058 |
| Yes | 0 (0.0%) | 3 (1.3%) | 3 (1.3%) | |
| Dialysis during the week before LT | 2 (0.4%) | 22 (9.6%) | 325 (10.0%) | <0.001 |
| Laboratory MELD score at LT | 15 (11, 19) | 23 (17, 30) | 22.7 (17, 30) | <0.001 |
| Albumin Level at LT | 3 (2.6, 3.5) | 2.9 (2.5, 3.4) | 2.9 (2.5, 3.4) | 0.014 |
| Previous abdominal surgery | 250 (53.9%) | 149 (64.8%) | 1963 (60.6%) | 0.007 |
| Portal vein thrombosis | 39 (8.4%) | 31 (13.5%) | 357 (11.0%) | 0.100 |
| UNOS/OPTN region where listed/ transplanted | ||||
| 1 | 32 (6.9%) | 3 (1.3%) | 89 (2.7%) | <0.001 |
| 2 | 80 (17.2%) | 37 (16.1%) | 289 (8.9%) | |
| 3 | 5 (1.1%) | 31 (13.5%) | 597 (18.4%) | |
| 4 | 23 (5.0%) | 8 (3.5%) | 338 (10.4%) | |
| 5 | 93 (20.0%) | 21 (9.1%) | 461 (14.2%) | |
| 6 | 1 (0.2%) | 3 (1.3%) | 119 (3.7%) | |
| 7 | 83 (17.9%) | 29 (12.6%) | 293 (9.1%) | |
| 8 | 30 (6.5%) | 17 (7.4%) | 239 (7.4%) | |
| 9 | 64 (13.8%) | 16 (7.0%) | 179 (5.5%) | |
| 10 | 28 (6.0%) | 41 (17.8%) | 361 (11.2%) | |
| 11 | 25 (5.4%) | 24 (10.4%) | 272 (8.4%) | |
| MELD exception points were given for HCC | 6 (1.3%) | 18 (7.8%) | 297 (9.2%) | <0.001 |
| Total days on waiting list/including inactive time | 170 (83, 371) | 120 (20, 380) | 111 (26, 373) | <0.001 |
| Time periods | ||||
| <2011 | 196 (42.2%) | 84 (36.5%) | 1446 (44.7%) | 0.089 |
| 2010–2019 | 205 (44.2%) | 110 (47.8%) | 1412 (43.6%) | |
| ≥2020 | 63 (13.6%) | 36 (15.7%) | 379 (11.7%) | |
| DONOR CHARACTERISTICS | ||||
| Age (years) | 36 (28, 45) | 39 (27, 53) | 45 (28, 58) | <0.001 |
| Gender, n (%) | ||||
| Female | 256 (55.2%) | 104 (45.2%) | 1564 (48.3%) | 0.011 |
| Male | 208 (44.8%) | 126 (54.8%) | 1673 (51.7%) | |
| Race, n (%) | ||||
| White | 372 (80.2%) | 148 (64.3%) | 2137 (66.0%) | <0.001 |
| Black | 14 (3.0%) | 48 (20.9%) | 502 (15.5%) | |
| Hispanic | 62 (13.4%) | 28 (12.2%) | 462 (14.3%) | |
| Asian | 6 (1.3%) | 5 (2.2%) | 75 (2.3%) | |
| Other | 10 (2.2%) | 1 (0.4%) | 61 (1.9%) | |
| DM, n (%) | 0 (0.0%) | 31 (13.5%) | 383 (11.8%) | <0.001 |
| BMI | 26.3 (23.8, 28.3) | 26.2 (23, 30.4) | 26.0 (22.7, 29.7) | 0.51 |
| Cause of death | ||||
| Anoxia | 0 (0.0%) | 125 (54.3%) | 820 (25.3%) | <0.001 |
| CVA | 0 (0.0%) | 45 (19.6%) | 1254 (38.7%) | |
| Head trauma | 0 (0.0%) | 55 (23.9%) | 1066 (32.9%) | |
| Other | 464 (100.0%) | 5 (2.2%) | 97 (3.0%) | |
| Cold ischemia time (hours) | 1.7 (1, 2.7) | 6.0 (4.5, 7.5) | 6.0 (4.8, 7.7) | <0.001 |
| Sharing region | ||||
| Local | 464 (100.0%) | 150 (65.2%) | 2116 (65.4%) | <0.001 |
| Regional | 0 (0.0%) | 51 (22.2%) | 822 (25.4%) | |
| National | 0 (0.0%) | 29 (12.6%) | 299 (9.2%) | |
| Donor–recipient match per BSA | ||||
| Too small | 0 (0.0%) | 3 (1.3%) | 61 (1.9%) | 0.022 |
| Appropriate size | 404 (87.1%) | 199 (86.5%) | 2834 (87.6%) | |
| Too large | 60 (12.9%) | 28 (12.2%) | 342 (10.6%) | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| RECIPIENT CHARACTERISTICS | ||||||
| Recipient Age | 1.32 | 1.23–1.42 | <0.001 | 1.28 | 1.20–1.38 | <0.001 |
| Male Gender | 1.31 | 1.13–1.52 | <0.001 | 1.26 | 1.08–1.47 | 0.003 |
| Recipient race (White ref.) | ||||||
| Black | 0.94 | 0.74–1.20 | 0.63 | |||
| Hispanic | 0.86 | 0.71–1.04 | 0.11 | |||
| Asian | 0.78 | 0.48–1.26 | 0.31 | |||
| Other | 0.90 | 0.52–1.56 | 0.71 | |||
| DM | 1.38 | 1.17–1.63 | <0.001 | 1.27 | 1.08–1.50 | 0.005 |
| Blood type (O ref.) | ||||||
| A | 1.09 | 0.95–1.25 | 0.22 | |||
| B | 1.13 | 0.93–1.36 | 0.21 | |||
| AB | 1.22 | 0.92–1.63 | 0.17 | |||
| BMI | 1.02 | 1.00–1.03 | 0.01 | 1.01 | 1.00–1.02 | 0.03 |
| Life Support | 1.04 | 0.54–2.00 | 0.91 | |||
| Dialysis during the week before LT | 1.46 | 1.18–1.79 | <0.001 | 1.51 | 1.23–1.87 | <0.001 |
| Laboratory MELD score at LT | 1.00 | 0.99–1.00 | 0.28 | |||
| Albumin level at LT | 1.02 | 0.94–1.11 | 0.64 | |||
| Previous abdominal surgery | 1.15 | 1.01–1.30 | 0.03 | |||
| Portal vein thrombosis | 1.28 | 1.04–1.56 | 0.02 | 1.25 | 1.02–1.53 | 0.03 |
| Region (Region 1 ref.) | ||||||
| 2 | 1.08 | 0.74–1.55 | 0.70 | 1.36 | 1.13–1.63 | 0.001 |
| 3 | 0.79 | 0.55–1.14 | 0.21 | |||
| 4 | 0.88 | 0.60–1.29 | 0.51 | |||
| 5 | 0.67 | 0.46–0.98 | 0.04 | |||
| 6 | 0.58 | 0.36–0.93 | 0.02 | |||
| 7 | 0.78 | 0.53–1.13 | 0.19 | |||
| 8 | 0.63 | 0.42–0.94 | 0.02 | |||
| 9 | 0.84 | 0.56–1.25 | 0.38 | |||
| 10 | 0.83 | 0.57–1.21 | 0.34 | |||
| 11 | 0.83 | 0.56–1.23 | 0.35 | |||
| MELD exception points were given for HCC | 1.35 | 1.10–1.65 | 0.01 | |||
| Time on waiting list | 1.00 | 1.00–1.00 | 0.94 | |||
| Period (2002–2010 ref.) | ||||||
| 2011–2019 | 0.86 | 0.74–0.99 | 0.04 | 0.84 | 0.74–0.96 | 0.01 |
| 2020–2021 | 1.11 | 0.77–1.58 | 0.58 | |||
| DONOR CHARACTERISTICS | ||||||
| Donor Age | 1.01 | 1.00–1.01 | <0.001 | 1.00 | 1.00–1.01 | 0.007 |
| Male Gender | 1.11 | 0.99–1.26 | 0.09 | |||
| Donor race (White ref.) | ||||||
| Black | 1.21 | 1.01–1.44 | 0.04 | |||
| Hispanic | 1.03 | 0.86–1.25 | 0.73 | |||
| Asian | 1.25 | 0.84–1.84 | 0.27 | |||
| Other | 1.58 | 1.04–2.40 | 0.03 | 1.58 | 1.05–2.40 | 0.03 |
| DM | 1.06 | 1.00–1.13 | 0.06 | |||
| BMI | 1.01 | 1.00–1.02 | 0.15 | |||
| Living Donor | 0.79 | 0.64–0.97 | 0.02 | |||
| Graft type (LDLT ref.) | ||||||
| DBD | 1.63 | 1.19–2.22 | <0.001 | |||
| DCD | 1.25 | 1.02–1.53 | 0.03 | |||
| Cause of death (Anoxia ref.) | ||||||
| CVA | 1.12 | 0.94–1.33 | 0.20 | |||
| Head trauma | 1.03 | 0.86–1.23 | 0.77 | |||
| Other | 0.88 | 0.71–1.10 | 0.28 | |||
| Cold ischemia time | 1.02 | 1.01–1.04 | 0.001 | |||
| Sharing region (Local ref.) | ||||||
| Regional | 0.96 | 0.83–1.13 | 0.65 | |||
| National | 1.58 | 1.27–1.97 | 0.001 | 1.38 | 1.11–1.73 | 0.004 |
| Donor–Recipient match as per BSA (Appropriate ref.) | ||||||
| Too small | 1.35 | 0.87–2.11 | 0.18 | |||
| Too large | 0.96 | 0.78–1.18 | 0.70 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| RECIPIENT CHARACTERISTICS | ||||||
| Recipient Age | 1.15 | 1.07–1.22 | <0.001 | 1.12 | 1.05–1.93 | 0.001 |
| Male Gender | 1.29 | 1.11–1.49 | 0.001 | 1.24 | 1.08–1.44 | 0.003 |
| Recipient race (White ref.) | ||||||
| Black | 0.93 | 0.74–1.18 | 0.56 | |||
| Hispanic | 0.81 | 0.68–0.97 | 0.03 | |||
| Asian | 0.75 | 0.48–1.19 | 0.22 | |||
| Other | 0.92 | 0.55–1.54 | 0.76 | |||
| DM | 1.28 | 1.09–1.50 | 0.003 | 1.22 | 1.04–1.44 | 0.01 |
| Blood type (O ref.) | ||||||
| A | 1.08 | 0.95–1.23 | 0.22 | |||
| B | 1.18 | 0.99–1.41 | 0.07 | |||
| AB | 1.21 | 0.92–1.59 | 0.18 | |||
| BMI | 1.01 | 1.00–1.02 | 0.03 | |||
| Life support | 1.23 | 0.68–2.23 | 0.49 | |||
| Dialysis during the week before LT | 1.36 | 1.11–1.66 | 0.003 | 1.46 | 1.19–1.78 | <0.001 |
| Laboratory MELD score at LT | 0.99 | 0.99–1.00 | 0.11 | |||
| Albumin level at LT | 1.04 | 0.96–1.13 | 0.36 | |||
| Previous abdominal surgery | 1.10 | 0.98–1.24 | 0.12 | |||
| Portal vein thrombosis | 1.18 | 0.98–1.44 | 0.09 | |||
| Region (Region 1 ref.) | ||||||
| 2 | 1.12 | 0.79–1.59 | 0.54 | 1.35 | 1.14–1.61 | 0.001 |
| 3 | 0.80 | 0.56–1.13 | 0.20 | |||
| 4 | 0.87 | 0.61–1.26 | 0.47 | |||
| 5 | 0.75 | 0.52–1.07 | 0.11 | |||
| 6 | 0.59 | 0.37–0.94 | 0.03 | |||
| 7 | 0.84 | 0.59–1.21 | 0.35 | |||
| 8 | 0.65 | 0.44–0.95 | 0.03 | |||
| 9 | 0.91 | 0.62–1.33 | 0.62 | |||
| 10 | 0.86 | 0.60–1.24 | 0.42 | |||
| 11 | 0.84 | 0.58–1.22 | 0.35 | |||
| MELD exception points were given for HCC | 1.22 | 1.00–1.50 | 0.05 | |||
| Time on waiting list | 1.00 | 1.00–1.00 | 0.59 | |||
| Period (2002–2010 ref.) | ||||||
| 2011–2019 | 0.80 | 0.69–0.92 | 0.002 | 0.79 | 0.68–0.90 | 0.001 |
| 2020–2021 | 0.95 | 0.68–1.31 | 0.74 | |||
| Donor characteristics | ||||||
| Donor age | 1.01 | 1.00–1.01 | <0.001 | 1.01 | 1.00–1.01 | <0.001 |
| Male gender | 1.07 | 0.95–1.21 | 0.24 | |||
| Donor race (White ref.) | ||||||
| Black | 1.18 | 1.00–1.40 | 0.05 | |||
| Hispanic | 0.97 | 0.81–1.16 | 0.71 | |||
| Asian | 1.10 | 0.74–1.62 | 0.64 | |||
| Other | 1.35 | 0.89–2.05 | 0.15 | |||
| DM | 1.06 | 0.99–1.12 | 0.08 | |||
| BMI | 1.01 | 1.00–1.02 | 0.19 | |||
| Living donor | 0.94 | 0.78–1.13 | 0.53 | |||
| Graft type (LDLT ref.) | ||||||
| DBD | 1.34 | 1.00–1.79 | 0.05 | |||
| DCD | 1.04 | 0.87–1.26 | 0.64 | |||
| Cause of death (Anoxia ref.) | ||||||
| CVA | 1.14 | 0.96–1.35 | 0.13 | |||
| Head trauma | 1.04 | 0.88–1.24 | 0.63 | |||
| Other | 1.04 | 0.85–1.28 | 0.70 | |||
| Cold ischemia time | 1.02 | 1.01–1.04 | 0.01 | |||
| Sharing region (Local ref.) | ||||||
| Regional | 0.94 | 0.81–1.09 | 0.39 | |||
| National | 1.47 | 1.19–1.82 | <0.001 | 1.28 | 1.04–1.59 | 0.02 |
| Donor–Recipient match as per BSA (Appropriate ref.) | ||||||
| Too small | 1.36 | 0.89–2.07 | 0.16 | |||
| Too large | 0.94 | 0.77–1.15 | 0.55 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Morales, E.; Ismail, M.; Barba Bernal, R.; Abboud, Y.; Sierra, L.; Marenco-Flores, A.; Goyes, D.; Saberi, B.; Patwardhan, V.; Bonder, A. Two Decades of Liver Transplants for Primary Biliary Cholangitis: A Comparative Study of Living Donors vs. Deceased Donor Liver Transplantations. J. Clin. Med. 2023, 12, 6536. https://doi.org/10.3390/jcm12206536
Medina-Morales E, Ismail M, Barba Bernal R, Abboud Y, Sierra L, Marenco-Flores A, Goyes D, Saberi B, Patwardhan V, Bonder A. Two Decades of Liver Transplants for Primary Biliary Cholangitis: A Comparative Study of Living Donors vs. Deceased Donor Liver Transplantations. Journal of Clinical Medicine. 2023; 12(20):6536. https://doi.org/10.3390/jcm12206536
Chicago/Turabian StyleMedina-Morales, Esli, Mohamed Ismail, Romelia Barba Bernal, Yazan Abboud, Leandro Sierra, Ana Marenco-Flores, Daniela Goyes, Behnam Saberi, Vilas Patwardhan, and Alan Bonder. 2023. "Two Decades of Liver Transplants for Primary Biliary Cholangitis: A Comparative Study of Living Donors vs. Deceased Donor Liver Transplantations" Journal of Clinical Medicine 12, no. 20: 6536. https://doi.org/10.3390/jcm12206536