Bile Duct Lithiasis Mimicking a Perihilar Cholangiocarcinoma—An Endless Dilemma: A Case Report
Abstract
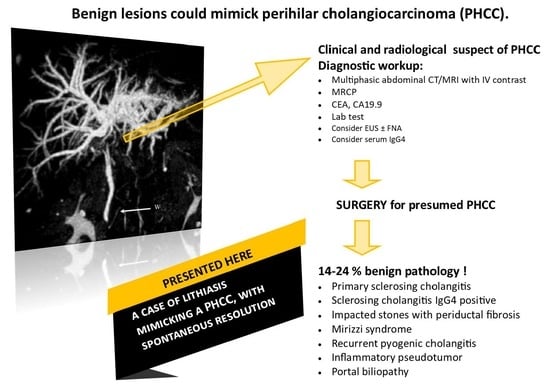
:1. Introduction
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Gelrud, A.; Agarwal, B. Biliary strictures: Diagnostic considerations and approach. Gastroenterol. Rep. 2015, 3, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farges, O.; Regimbeau, J.M.; Fuks, D.; Le Treut, Y.P.; Cherqui, D.; Bachellier, P.; Mabrut, J.Y.; Adham, M.; Pruvot, F.R.; Gigot, J.F. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br. J. Surg. 2012, 100, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, G.; Giuliante, F.; Ardito, F.; Giovannini, I.; Aldrighetti, L.; Belli, G.; Bresadola, F.; Calise, F.; Valle, R.D.; D’Amico, D.F.; et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: Results of an italian multicenter analysis of 440 patients. Arch. Surg. 2012, 147, 26–34. [Google Scholar] [CrossRef]
- NCCN. NCCN Guidelines for Biliary Tract Cancers, Version 2.2023; National Comprehensive Cancer Network® (NCCN®): Fort Washington, PA, USA, 5 October 2023.
- Ringe, K.I.; Wacker, F. Radiological diagnosis in cholangiocarcinoma: Application of computed tomography, magnetic resonance imaging, and positron emission tomography. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, D.; Kloek, J.J.; Kate, F.J.W.T.; Rauws, E.A.J.; Busch, O.R.C.; Gouma, D.J.; van Gulik, T.M. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br. J. Surg. 2008, 95, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, W.T.; Jonas, S. Spectrum of benign lesions mimicking a malignant stricture at the liver hilum. Rev. Recent Clin. Trials 2009, 4, 185–194. [Google Scholar] [CrossRef]
- Agha, R.A.; Franchi, T.; Sohrabi, C.; Mathew, G.; SG Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int. J. Surg. 2020, 84, 226–230. [Google Scholar] [CrossRef]
- Sarawagi, R.; Sundar, S.; Raghuvanshi, S.; Gupta, S.K.; Jayaraman, G. Common and Uncommon Anatomical Variants of Intrahepatic Bile Ducts in Magnetic Resonance Cholangiopancreatography and its Clinical Implication. Pol. J. Radiol. 2016, 81, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Läuffer, J.M.; Baer, H.U.; Schajor, M.; Halter, F.; Büchler, M.W. Choledocholithiasis at the hepatic confluence mimicking a hilar cholangiocarcinoma. Hepato-Gastroenterology 1998, 45, 2339–2343. [Google Scholar]
- Corvera, C.U.; Blumgart, L.H.; Darvishian, F.; Klimstra, D.S.; DeMatteo, R.; Fong, Y.; D’Angelica, M.; Jarnagin, W.R. Clinical and Pathologic Features of Proximal Biliary Strictures Masquerading as Hilar Cholangiocarcinoma. J. Am. Coll. Surg. 2005, 201, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.; Clarke, D.; Currie, E.; Madhavan, K.; Parks, R.; Garden, O. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgery 2003, 1, 32–38. [Google Scholar] [CrossRef]
- Tanisaka, Y.; Mizuide, M.; Fujita, A.; Ogawa, T.; Suzuki, M.; Katsuda, H.; Saito, Y.; Miyaguchi, K.; Tashima, T.; Mashimo, Y.; et al. Diagnostic Process Using Endoscopy for Biliary Strictures: A Narrative Review. J. Clin. Med. 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tejaswi, S. Clinical impact of digital cholangioscopy in management of indeterminate biliary strictures and complex biliary stones: A single-center study. Ther. Adv. Gastrointest. Endosc. 2019, 12, 2631774519853160. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Morais, R.; Vilas-Boas, F.; Rodrigues-Pinto, E.; Lopes, J.; Carneiro, F.; Macedo, G. Brush Cytology Performance for the Assessment of Biliopancreatic Strictures. Acta Cytol. 2020, 64, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Hu, S.; Dai, W.; Wu, S.; Kong, J. Dilemma of the differential diagnosis of hilar cholangiocarcinoma and benign diseases: A single-center retrospective study. Carcinogenesis 2021, 42, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Boškoski, I.; Familiari, P.; Tringali, A.; Cesaro, P.; Perri, V. Update in Biliary Endoscopy. Dig. Dis. 2011, 29, 3–8. [Google Scholar] [CrossRef]
- Inoko, K.; Tsuchikawa, T.; Noji, T.; Kurashima, Y.; Ebihara, Y.; Tamoto, E.; Nakamura, T.; Murakami, S.; Okamura, K.; Shichinohe, T.; et al. Hilar cholangiocarcinoma with intratumoral calcification: A case report. World J. Gastroenterol. 2015, 21, 10926–10930. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Al-Harthi, N.; Heathcote, E.J. Review: Current status of therapy in autoimmune liver disease. Ther. Adv. Gastroenterol. 2009, 2, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver 2019, 13, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Sarcognato, S.; Sacchi, D.; Grillo, F.; Cazzagon, N.; Fabris, L.; Cadamuro, M.; Cataldo, I.; Covelli, C.; Mangia, A.; Guido, M. Autoimmune biliary diseases: Primary biliary cholangitis and primary sclerosing cholangitis. Pathologica 2021, 113, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Kawa, S.; Uehara, T.; Ochi, Y.; Takayama, M.; Komatsu, K.; Muraki, T.; Umino, J.; Kiyosawa, K.; Miyagawa, S. Immunoglobulin G4-related lymphoplasmacytic sclerosing cholangitis that mimics infiltrating hilar cholangiocarcinoma: Part of a spectrum of autoimmune pancreatitis? Gastrointest. Endosc. 2005, 62, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Nagasaka, T.; Suzuki, K.; Satake, H.; Ota, T.; Naganawa, S. Lymphoplasmacytic sclerosing cholangitis: Assessment of clinical, CT, and pathological findings. Clin. Radiol. 2009, 64, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Ntinas, A.; Kardassis, D.; Miliaras, D.; Tsinoglou, K.; Dimitriades, A.; Vrochides, D. Inflammatory pseudotumor of the liver: A case report and review of the literature. J. Med. Case Rep. 2011, 215, 196. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.; Hwang, S.; Ahn, C.-S.; Kim, K.-H.; Moon, D.-B.; Ha, T.-Y.; Song, G.-W.; Jung, D.-H.; Hong, S.-M. Clinicopathological features and post-resection outcomes of inflammatory pseudotumor of the liver. Ann. Hepato-Biliary-Pancreatic Surg. 2021, 25, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Menias, C.O.; Surabhi, V.R.; Prasad, S.R.; Wang, H.L.; KNC, V.R.N. Mimics of Cholangiocarcinoma: Spectrum of Disease. Radiographics 2008, 28, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Lesurtel, M.; Belghiti, J. Is preoperative histological diagnosis necessary before referral to major surgery for cholangiocarcinoma? HPB 2008, 10, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Toy, E.; Balasubramanian, S.; Selmi, C.; Li, C.-S.; Bowlus, C.L. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011, 11, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floreani, A.; Okazaki, K.; Uchida, K.; Gershwin, M.E. IgG4-related disease: Changing epidemiology and new thoughts on a multisystem disease. J. Transl. Autoimmun. 2020, 4, 100074. [Google Scholar] [CrossRef]


| Lab Tests | |||||||
|---|---|---|---|---|---|---|---|
| Total bilirubin (mg/dL) | 7 | 7.5 | 3.2 | 2 | 1.2 | ||
| Direct bilirubin (mg/dL) | 5.5 | 6.9 | 2.7 | 1.8 | 1.2 | ||
| S-GPT (U/L) | 325 | 248 | 80 | 55 | 49 | ||
| S-GOT (U/L) | 145 | 123 | 24 | 31 | 30 | ||
| ALP (U/L) | 324 | 387 | 248 | 224 | 161 | ||
| Lipase (U/L)) | 41 | 3215 | 82 | 77 | 37 | ||
| PT-INR | 1.36 | 1.51 | 1.32 | 1.18 | 1.13 | ||
| Hb (g/dL) | 13.5 | 14.3 | 11.9 | 12.3 | 12.7 | ||
| Leucocytes (cell/mcl) | 10,510 | 12,710 | 12,640 | 13,540 | 5070 | ||
| CRP (mg/dL) | 0.96 | 2.8 | 26.2 | 17.96 | 0.52 | ||
| CA19-9 (U/mL) | 281.5 | 35 | 8.6 | ||||
| Imaging performed | Abdominal MRI + MRCP | Abdominal CT | Abdominal CT | Abdominal MRI + MRCP | |||
| Events | Admission to community hospital | Admission to our Dept. | Acute pancreatitis | Resolution of the scenario | |||
| Timeline | Day 0 | 4 days later | 6 days | 7 days | 10 days | 12 days | 21 days |
| Primary Sclerosing Cholangitis | Lymphoplasmacytic Sclerosing Cholangitis-Sclerosing Cholangitis IgG4 Positive (as Part of Systemic IgG4-Related Disease) | Recurrent Pyogenic Cholangitis | Inflammatory Pseudotumor (IPT) | Impacted Stones with Periductal Fibrosis | Mirizzi Syndrome | Portal Biliopathy | |
|---|---|---|---|---|---|---|---|
| Clinincal presentation | Jaundice, pruritus, abdominal pain. | Jaundice, pruritus, abdominal pain. | Jaundice, fever, abdominal pain. | Asympthomatic or abdominal pain and fever. | Jaundice, fever, abdominal pain. | Jaundice, ± fever, abdominal pain. | Often asympthomatic. Patient history of extrahepatic portal vein thrombosis is the most common cause of portal biliopathy; rare in cirrhosis, portal vein fibrosis without cirrhosis and congenital hepatic fibrosis. |
| Epidemiology | Age at diagnosis 44.2 y.o. ± 17.4 (11–81) *. The age-adjusted incidence rate for males was numerically greater than females. Patients are usually nonsmokers, and about 2/3 have a coexistent IBD (75% ulcerative colitis). | Middle to upper age, with an onset at 50–70 years, Male:female ratio = 3:7. | More frequent between the third and fifth decades. | Incidence correlates to gallbladder stones. | |||
| Laboratory test | Elevated ALP, GGT, bilirubin. Different non-specific autoantibodies correlate with PSC, such as P-ANCA, ANA, anti-smooth muscle autoantibodies | Elevated serum IgG4 concentration (≥135 mg/dL). CA19-9 can be elevated. | Elevated inflammatory markers (including erythrocyte sedimentation rate, C-reactive protein, and leukocyte count) are common. CA19-9 usually normal. | Elevated ALP, GGT, bilirubin. ± elevated inflammatory markers. CA19-9 can be elevated because of jaundice. | Elevated ALP, GGT, bilirubin, GOT, GPT. CA19-9 can be elevated. | ||
| Appropriate imaging modality and features | MRCP, ERC. Beaded appearance, pruned tree appearance, and band-like stricture. | MRCP. Diffuse or segmental narrowing of the intrahepatic and/or extrahepatic bile duct, associated with the thickening of the bile duct wall. | MRCP. Intraductal calculi and bile duct strictures. | CT-scan, MRI. The CT-scan: lesions with variable c.e., may present as hypovascular with delayed enhancement because of fibrosis. The MRI may produce hypointense on T1 sequences with moderate-to-high hyperintense on T2 sequences. | CT scan (scarce sensitivity for non-calcific stones) and MRCP. | CT scan, MRCP. MRCP most accurate, shows an extrinsic narrowing of the common hepatic duct, a gallstone in the cystic duct, dilation of the intrahepatic and common hepatic ducts, with a normal common bile duct. | CT scan and portal MR and MRCP. Show portal cavernoma, paracholedochal and/or epicholedochal dilations, portosystemic shunts and abnormal morphology of the bile duct. |
| Specific investigation | Cholangiography, liver biopsy for doubtful cases. | Elevated serum IgG4 concentration (≥135 mg/dL). | Exclusion diagnosis. Percutaneous liver biopsy. | ||||
| Histo-pathological examination |
Obliterative, non-suppurative cholangitis with substantial periductular fibrosis, referred to as “onion-skin fibrosis”. | Marked lymphocytic and plasmacytic infiltration and fibrosis. Infiltration of IgG4-positive plasma cells (>10 cells per high-power field). Storiform fibrosis. Obliterative phlebitis. | Chronic, recurrent infections from parasites predispose to the development of pigmented calculi, cholangitic abscesses, and inflammatory strictures. | Inflammatory infiltrate consisting of lymphocytes, plasma cells, and histiocytes admixed with a variable proportion of fibroblasts and myofibroblasts. | Acute or chronic cholecystitis. | ||
| Specific medical intervention | High dose of UDCA (20 mg/kg/d) improve liver function tests. | Prednisone 20–40 mg/d. | Improvement after antibiotics or after steroid administration. | Treat underlying disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccino, M.; Hoxhaj, I.; Grossi, U.; Romano, M.; Brizzolari, M.; Scopelliti, M.; Finotti, M.; Zanus, G. Bile Duct Lithiasis Mimicking a Perihilar Cholangiocarcinoma—An Endless Dilemma: A Case Report. J. Clin. Med. 2023, 12, 5104. https://doi.org/10.3390/jcm12155104
Piccino M, Hoxhaj I, Grossi U, Romano M, Brizzolari M, Scopelliti M, Finotti M, Zanus G. Bile Duct Lithiasis Mimicking a Perihilar Cholangiocarcinoma—An Endless Dilemma: A Case Report. Journal of Clinical Medicine. 2023; 12(15):5104. https://doi.org/10.3390/jcm12155104
Chicago/Turabian StylePiccino, Marco, Ilda Hoxhaj, Ugo Grossi, Maurizio Romano, Marco Brizzolari, Michele Scopelliti, Michele Finotti, and Giacomo Zanus. 2023. "Bile Duct Lithiasis Mimicking a Perihilar Cholangiocarcinoma—An Endless Dilemma: A Case Report" Journal of Clinical Medicine 12, no. 15: 5104. https://doi.org/10.3390/jcm12155104