A Comparison of Observational Studies on Subfoveal Choroidal Thickness Measured with OCT according to the Level of Thyroid Eye Disease Activity—Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Statistical Analysis
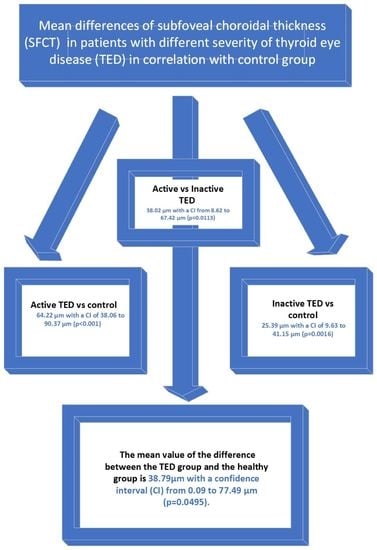
3. Results
3.1. The Subgroup Analysis
3.2. The Trim-and-Fill Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alajmi, S.; Alshehri, S.; Ekhzaimy, A. Graves’ Ophthalmopathy in the Setting of Primary Hypothyroidism. Cureus 2022, 14, e24954. [Google Scholar] [CrossRef] [PubMed]
- Boddu, N.; Jumani, M.; Wadhwa, V.; Bajaj, G.; Faas, F. Not All Orbitopathy Is Graves’: Discussion of Cases and Review of Literature. Front. Endocrinol. 2017, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Bahn, R.S. Graves’ Ophthalmopathy. N. Engl. J. Med. 2010, 362, 726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, N.M.; Rajaii, F. Current Understanding of the Progression and Management of Thyroid Associated Orbitopathy: A Systematic Review. Ophthalmol. Ther. 2020, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Neag, E.J.; Smith, T.J. 2021 update on thyroid-associated ophthalmopathy. J. Endocrinol. Investig. 2022, 45, 235–259. [Google Scholar] [CrossRef]
- Lee, A.C.H.; Kahaly, G.J. Pathophysiology of thyroid-associated orbitopathy. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 37, 101620. [Google Scholar] [CrossRef]
- Kofler, S.; Nickel, T.; Weis, M. Role of cytokines in cardiovascular diseases: A focus on endothelial responses to inflammation. Clin. Sci. 2005, 108, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; Ayvaz, G.; et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Barrio-Barrio, J.; Sabater, A.L.; Bonet-Farriol, E.; Velázquez-Villoria, Á.; Galofré, J.C. Graves’ Ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J. Ophthalmol. 2015, 2015, 249125. [Google Scholar] [CrossRef] [Green Version]
- Goel, R.; Shah, S.; Sundar, G.; Arora, R.; Gupta, S.; Khullar, T. Orbital and ocular perfusion in thyroid eye disease. Surv. Ophthalmol. 2023, 68, 481–506. [Google Scholar] [CrossRef]
- Liberati, M.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Basural, E.; Ozturk, H.E. Comparison of choroidal thickness in patients with active and stable thyroid eye disease. Arq. Bras. Oftalmol. 2019, 82, 124–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeter, V.; Koçak, N.; Subaşı, M.; Parlak, U. Choroidal vascularity index in thyroid-associated ophthalmopathy. Can. J. Ophthalmol. 2021, 58, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ozturk Karabulut, G.; Fazil, K.; Ozturker, C.; Gunaydin, Z.K.; Altan, C.; İnal, A.; Taskapili, M.; Kaynak, P. Do ocular pulse amplitude and choroidal thickness change in patients with thyroid eye disease? Orbit 2019, 38, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kurt, M.M.; Akpolat, C.; Evliyaoglu, F.; Yilmaz, M.; Ordulu, F. Evaluation of Retinal Neurodegeneration and Choroidal Thickness in Patients with Inactive Graves’ Ophthalmopathy. Klin. Monbl. Augenheilkd. 2021, 238, 797–802. [Google Scholar] [CrossRef]
- Ceylanoglu, K.S.; Eser, N.A.; Sen, E.M. Choroidal structural evaluation in inactive Graves’ ophthalmopathy. Photodiagnosis Photodyn. Ther. 2022, 39, 103012. [Google Scholar] [CrossRef]
- Yıldırım, G.; Şahlı, E.; Alp, M.N. Evaluation of the effect of proptosis on choroidal thickness in graves’ ophthalmopathy. Turkish J. Ophthalmol. 2020, 50, 221–227. [Google Scholar] [CrossRef]
- Çalışkan, S.; Acar, M.; Gürdal, C. Choroidal Thickness in Patients with Graves’ Ophthalmopathy. Curr. Eye Res. 2017, 42, 484–490. [Google Scholar] [CrossRef]
- Lai, F.H.P.; Iao, T.W.U.; Ng, D.S.C.; Young, A.L.; Leung, J.; Au, A.; Ko, S.T.C.; Chong, K.K.L. Choroidal thickness in thyroid-associated orbitopathy. Clin. Exp. Ophthalmol. 2019, 47, 918–924. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Zhang, J.M.; Tian, C.L.; Ji, Y.N.; Zhang, L.L. Analysis of different choroidal stratification thickness under macular fovea in patients with early graves ophthalmopathy. Int. Eye Sci. 2019, 19, 2150–2152. [Google Scholar] [CrossRef]
- Yu, L.; Jiao, Q.; Cheng, Y.; Zhu, Y.; Lin, Z.; Shen, X. Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography. BMC Ophthalmol. 2020, 20, 421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Song, Y.; Cai, Q.; Zhou, Y.; Li, J. A study on observing the central macular choroidal thickness of thyroid-associated ophthalmopathy patients with spectral-domain optical coherence tomography. Zhonghua Yan Ke Za Zhi. 2018, 54, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bruscolini, A.; La Cava, M.; Gharbiya, M.; Sacchetti, M.; Restivo, L.; Nardella, C.; Marenco, M.; Lambiase, A. Management of patients with Graves’ disease and orbital involvement: Role of spectral domain optical coherence tomography. J. Immunol. Res. 2018, 2018, 1454616. [Google Scholar] [CrossRef] [Green Version]
- Casini, G.; Marinò, M.; Rubino, M.; Licari, S.; Covello, G.; Mazzi, B.; Ionni, I.; Rocchi, R.; Sframeli, A.T.; Figus, M.; et al. Retinal, choroidal and optic disc analysis in patients with Graves’ disease with or without orbitopathy. Int. Ophthalmol. 2020, 40, 2129–2137. [Google Scholar] [CrossRef]
- Loiudice, P.; Pellegrini, M.; Marinò, M.; Mazzi, B.; Ionni, I.; Covello, G.; Figus, M.; Nardi, M.; Casini, G. Choroidal vascularity index in thyroid-associated ophthalmopathy: A cross-sectional study. Eye Vis. 2021, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Del Noce, C.; Vagge, A.; Nicolò, M.; Traverso, C.E. Evaluation of choroidal thickness and choroidal vascular blood flow in patients with thyroid-associated orbitopathy (TAO) using SD-OCT and Angio-OCT. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1103–1107. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.; Baek, S. Increase of central foveal and temporal choroidal thickness in patients with inactive thyroid eye disease. BMC Ophthalmol. 2021, 21, 32. [Google Scholar] [CrossRef]
- Gonçalves, A.C.P.; Gebrim, E.M.M.S.; Monteiro, M.L.R. Imaging studies for diagnosing Graves’ orbitopathy and dysthyroid optic neuropathy. Clinics 2012, 67, 1327. [Google Scholar] [CrossRef]
- Walasik-Szemplińska, D.; Pauk-Domańska, M.; Sanocka, U.; Sudoł-Szopińska, I. Doppler imaging of orbital vessels in the assessment of the activity and severity of thyroid-associated orbitopathy. J. Ultrason. 2015, 15, 388. [Google Scholar] [CrossRef]
- Monteiro, M.L.R.; Moritz, R.B.S.; Angotti-Neto, H.; Benabou, J.E. Color Doppler imaging of the superior ophthalmic vein in patients with Graves’ orbitopathy before and after treatment of congestive disease. Clinics 2011, 66, 1329. [Google Scholar]
- Tuerksever, C.; Pruente, C.; Hatz, K. High frequency SD-OCT follow-up leading to up to biweekly intravitreal ranibizumab treatment in neovascular age-related macular degeneration. Sci. Rep. 2021, 11, 6816. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kuang, G.P.; Luo, D.X.; Lu, X.H. Choroidal thickness in pregnant women: A cross-sectional study. Int. J. Ophthalmol. 2016, 9, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Özkan, B.; Koçer, A.; Altintaş Karabaş, L.; Acar, A.Z.; Yüksel, N. Choroidal changes observed with enhanced depth imaging optical coherence tomography in patients with mild Graves orbitopathy. Eye 2016, 30, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Roskal-Wałek, J.; Laudańska-Olszewska, I.; Biskup, M.; Gierada, M.; Odrobina, D. Choroidal Thickness in Women with Uncomplicated Pregnancy: Literature Review. BioMed Res. Int. 2017, 2017, 5694235. [Google Scholar] [CrossRef] [Green Version]
- Chien, L.; Go, C.C.; Lahaie Luna, G.M.; Briceño, C.A. Changes in choroidal thickness and choroidal vascularity index in thyroid eye disease: A systematic review. Orbit 2022. [Google Scholar] [CrossRef]
- Dave, T.V.; Natarajan, R.; Reddy, R.U.; Kapoor, A.G.; Dave, V.P. Choroidal Thickness in Thyroid Eye Disease: Comparison With Controls and Application in Diagnosing Non-Inflammatory Active Disease. Cureus 2021, 13, e19779. [Google Scholar] [CrossRef]
- Dave, T.V.; Jonnadula, G.B.; Lanka, P.; Natarajan, R.; Dave, V.P. Choroidal vascularity index in thyroid eye disease: Comparison with controls and application in diagnosing non-inflammatory active disease. Orbit 2022, 41, 89–96. [Google Scholar] [CrossRef]
- Cagiltay, E.; Akay, F.; Demir, O.; Aydin, E.; Akmaz, B.; Pamuk, B. The Increment of Choroidal Thickness in Euthyroid Graves’ Ophthalmopathy: Is It an Early Sign of Venous Congestion? J. Ophthalmol. 2018, 2018, 5891531. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Q.; Larsen, M.; Munch, I.C. Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8438–8441. [Google Scholar] [CrossRef]
- Fazil, K.; Karabulut, G.O.; Alkin, Z. Evaluation of choroidal thickness and retinal vessel density in patients with inactive Graves’ orbitopathy. Photodiagnosis Photodyn. Ther. 2020, 32, 101898. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, Y.; Kang, L.; Gao, Y.; Zhang, J.; Wu, Y. Analysis in Choroidal Thickness in Patients with Graves’ Ophthalmopathy Using Spectral-Domain Optical Coherence Tomography. J. Ophthalmol. 2018, 2018, 3529395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, R.; Spaide, R.F. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Tsujikawa, A.; Matsumoto, A.; Hangai, M.; Ooto, S.; Yamashiro, K.; Akiba, M.; Yoshimura, N. Macular Choroidal Thickness and Volume in Normal Subjects Measured by Swept-Source Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4971–4978. [Google Scholar] [CrossRef]
- Gokmen, O.; Ozgur, G. The Effects of chronic smoking on retinal vascular densities and choroidal thicknesses measured by optical coherence tomography angiography. Eur. J. Ophthalmol. 2022, 33, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Öncül, H.; Çağlayan, M.; Fuat Alakus, M.; Yılmaz öncül, F.; Dag, U.; Arac, E. Evaluation of the subfoveal choroidal and outer retinal layer thickness in obese women. Clin. Exp. Optom. 2021, 104, 178–186. [Google Scholar] [CrossRef]









| Publication Title | Quantity of TED Group, F/M | Quantity of Control Group, F/M | Age of TED Group (Average ± SD) | Age of Control Group (Average ± SD) | SFCT in TED Group | SFCT in Control Group | OCT Method |
|---|---|---|---|---|---|---|---|
| Zhang, Dan Zhao, Jun Zhang, Juan Mei et al., Analysis of different choroidal stratification thickness under macular fovea in patients with early graves ophthalmopathy [20] | 31 (62 eyes) | 31 (62 eyes) | 37 1 ± 12 5 (19–58) | Age-matched to the study group | 315.79 ± 14.99 μm | 239.00 ± 12.67 μm | EDI OCT |
| Volkan Yeter, Nurullah Kocak, Mustafa Suba, et al., Choroidal vascularity index in thyroid-associated ophthalmopathy [13] | 53 (53 eyes), 30/23 | 53 (53 eyes), 30/23 | 46.7 ± 13.3 (19–70) | 46.9 ± 13.5 (19–70) | 324.0 ± 63.8 (165–461) μm | 289.3 ± 78.8 (126–432) μm | EDI SDOCT (Heidelberg) |
| Bruscolini Alice, La Cava Maurizio, Gharbiya Magda et al., Management of patients with Graves’ disease and orbital involvement: Role of spectral domain optical coherence tomography [23] | 18 (36 eyes), 10/8 | 18 (36 eyes), 11/7 | 44.1 ± 9.8 (24–57) | 44.2 ± 10.7 (26–60) | 399.2 ± 84.1 μm | 344.5 ± 88.1 μm | EDI SDOCT (Heidelberg Spectralis) |
| Casini Giamberto, Marinò Michele, Rubino Marisa et al., Retinal, choroidal and optic disc analysis in patients with Graves’ disease with or without orbitopathy [24] | 40 (80 eyes), 35/5 | 40 (80 eyes), 29/11 | 39.57 ± 5.60 (23–49) | 36.28 ± 5.79 (29–49) | 288 ± 88 μm | 287 ± 58 μm | EDI SDOCT (Heidelberg) |
| Pasquale Loiudice, Marco Pellegrini, Michele Marinò, et al., Choroidal vascularity index in thyroid-associated ophthalmopathy: a cross-sectional study [25] | 40 (80 eyes) | 40 (80 eyes) | 39.30 ± 4.54 (30–45) | 37.45 ± 4.44 (28–45) | 308.08 ± 73.37 μm | 288.90 ± 58.32 μm | EDI SDOCT (Heidelberg) |
| Joohyun Kim, Sumin Yoon and Sehyun Baek, Increase of central foveal and temporal choroidal thickness in patients with inactive thyroid eye disease [27] | 49 (49 eyes), 34/15 | 49 (49 eyes), 34/15 | 51.1 ± 13.2 | 50.2 ± 14.4 | 294.2 ± 71.4 μm (only inactive TED) | 261.1 ± 47.4 μm | (EDI) mode by cirrus HD-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA) |
| Frank H P Lai, Tiara W U Iao, Danny S C Ng, et al., Choroidal thickness in thyroid-associated orbitopathy [19] | 52 (104 eyes), 35/17 | 26 (52 eyes), 16/10 | 47.4 ± 13.2 | 45.2 ± 15.6 | 331.29 ± 83.67 μm | 287.50 ± 78.55 μm | EDI SDOCT (Spectralis; Heidelberg) |
| Chiara Del Noce, Aldo Vagge, Massimo Nicolò, et al., Evaluation of choroidal thickness and choroidal vascular blood flow in patients with thyroid-associated orbitopathy (TAO) using SD-OCT and Angio-OCT [26] | 18 (36 eyes), 14/4 | 18 (36 eyes), 11/7 | 26.5 ± 4.94 (19–74) | 26.5 ± 3.53 | 285.6275 ± 32.5 μm | 135.89 ± 19.8 μm | SD-OCT Topcon ImageNet 6 (DRI OCT Triton, Topcon Corporation). |
| Gamze Ozturk Karabulut, Korhan Fazil, Can Ozturker, et al., Do ocular pulse amplitude and choroidal thickness change in patients with thyroid eye disease? [14] | 38 (38 eyes), 27/11 | 38 (38 eyes), 22/16 | 49 ± 11.8 | 49.07 ± 11.9 | 313.6 ± 76.1 μm | 396.3 ± 95.3 μm | EDI SDOCT, (Spectralis; Heidelberg) |
| Muhammed M Kurt, Cetin Akpolat, Ferhat Evliyaoglu, et al., Evaluation of Retinal Neurodegeneration and Choroidal Thickness in Patients with Inactive Graves’ Ophthalmopathy [15] | 29 (58 eyes), 14/15 | 30 (60 eyes), 18/12 | 40.66 ± 11.83 | 42.60 ± 12.38 | 305.53 ± 87.93 μm (only inactive TED) | 270.4 7 ± 60.35 μm | SS-OCT (automated module of the Triton) |
| Kubra Serbest Ceylanoglu, Nazan Acar Eser, Emine Malkoc Sen, Choroidal structural evaluation in inactive Graves’ ophthalmopathy [16] | 56 (56 eyes), 30/26 | 64 (64 eyes, 34/30) | 39.5 ± 11.4 | 42.2 ± 5.6 | 311.9 ± 30.0 μm (only inactive TED) | 295.4 ± 38.1 μm | EDI SDOCT Heidelberg Spectralis |
| Publication Title | Quantity of Active TED Group (F/M) | Quantity of Inactive TED Group (F/M) | Age of Active TED Group | Age of Inactive TED Group | SFCT Active TED | SFCT Inactive TED | OCT Method |
|---|---|---|---|---|---|---|---|
| Volkan Yeter, Nurullah Kocak, Mustafa Suba, et al., Choroidal vascularity index in thyroid-associated ophthalmopathy [13] | 17 eyes | 36 eyes | 366.5 ± 63.6 (247–461) μm | 308.4 ± 53.9 (165–396) μm | EDI SDOCT (Heidelberg) | ||
| Bruscolini Alice, La Cava Maurizio, Gharbiya Magda et al., Management of patients with Graves’ disease and orbital involvement: Role of spectral domain optical coherence tomography [23] | 8 (16 eyes) | 10 (20 eyes) | 436.2 ± 97.5 μm | 369.6 ± 89.3 μm | EDI SDOCT (Heidelberg Spectralis) | ||
| Yıldırım Gamze, Şahlı Esra, Alp Mehmet Numan, Evaluation of the effect of proptosis on choroidal thickness in graves’ ophthalmopathy [17] | 9 (9 eyes) | 41 (41 eyes) | 249.1 ± 45.4 μm | 318.6 ± 61.0 μm | EDI HD-OCT (Cirrus, Carl Zeiss Meditec Inc., Dublin, CA, USA). | ||
| Adem Gul, Ekrem Basural, Hilal Eser Ozturk, Comparison of choroidal thickness in patients with active and stable thyroid eye disease [12] | 24 (24 eyes) (15/9) | 23 (23 eyes) (19/4) | 40.7 ± 11.8 (22–60) | 43.7 ± 11.9 (18–56) | 304.79 ± 75.19 μm | 262.04 ± 63.46 μm | EDI HD-OCT (Cirrus, Carl Zeiss Meditec Inc., Dublin, CA, USA). |
| Zhu Y, Song Y, Cai Q, et al., A study on observing the central macular choroidal thickness of thyroid-associated ophthalmopathy patients with spectral-domain optical coherence tomography [22] | 11 (22 eyes) | 21 (42 eyes) | 391.27 ± 33.89 μm | 317.31 ± 29.62 μm | SD-OCT | ||
| Lanchu Yu, Qin Jiao, Yu Cheng, Yanji Zhu, Zhongjing Lin, Xi Shen, Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography [21] | 20 (20 eyes) (12/8) | 33 (33 eyes) (27/6) | 43.5 ± 11.5 | 39.3 ± 11.3 | 304 ± 41 μm | 299 ± 45 μm | HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA). The |
| Sinan Çalışkan, Mutlu Acar, Canan Gürdal, Choroidal Thickness in Patients with Graves’ Ophthalmopathy [18] | 14 (28 eyes, 12/2) | 24 (48 eyes, 20/4) | 46.7 ± 14.3 (23–65) | 40.5 ± 10.1 (25–56) | 395.84 ± 9.68 μm | 319.76 ± 7.07 μm | SD-OCT (RTVue-XR, software version 6.1, Optovue, Inc., Fremont, CA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik-Jagodzińska, M.; Sobol, M.; Turno-Kręcicka, A. A Comparison of Observational Studies on Subfoveal Choroidal Thickness Measured with OCT according to the Level of Thyroid Eye Disease Activity—Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4720. https://doi.org/10.3390/jcm12144720
Kowalik-Jagodzińska M, Sobol M, Turno-Kręcicka A. A Comparison of Observational Studies on Subfoveal Choroidal Thickness Measured with OCT according to the Level of Thyroid Eye Disease Activity—Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(14):4720. https://doi.org/10.3390/jcm12144720
Chicago/Turabian StyleKowalik-Jagodzińska, Małgorzata, Maria Sobol, and Anna Turno-Kręcicka. 2023. "A Comparison of Observational Studies on Subfoveal Choroidal Thickness Measured with OCT according to the Level of Thyroid Eye Disease Activity—Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 14: 4720. https://doi.org/10.3390/jcm12144720