Characteristics of Preoperative Arteriosclerosis Evaluated by Cardio-Ankle Vascular Index in Patients with Osteoarthritis before Total Knee Arthroplasty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement of CAVI
2.2. Reproducibility
2.3. Statistical Analysis
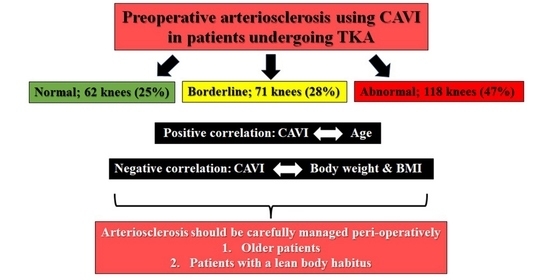
3. Results
4. Discussion
5. Conclusions
- The patient characteristics that warrant special attention to AS intraoperatively and immediately postoperatively are a lean body habitus (low BMI) and advanced age.
- Future studies based on the accumulation of preoperative CAVI data in patients with osteoarthritis who have various backgrounds, including patients with an ASA score of ≥III, are essential to more practically evaluate the impact of end-stage osteoarthritis on AS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, L.K.; Kendzerska, T.; Waugh, E.J.; Hawker, G.A. Impact of osteoarthritis on difficulty walking: A population-based study. Arthritis Care Res. 2018, 70, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendzerska, T.; Jüni, P.; King, L.K.; Croxford, R.; Stanaitis, I.; Hawker, G.A. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: A population-based cohort study. Osteoarthr. Cartil. 2017, 25, 1771–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, T.; Ito, H. Arterial stiffness in health and disease: The role of cardio-ankle vascular index. J. Cardiol. 2021, 78, 493–501. [Google Scholar] [CrossRef]
- Bierma-Zeinstra, S.M.A.; Waarsing, J.H. The role of atherosclerosis in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 613–633. [Google Scholar] [CrossRef]
- Hussain, S.M.; Dawson, C.; Wang, Y.; Tonkin, A.M.; Chou, L.; Wluka, A.E.; Cicuttini, F.M. Vascular pathology and osteoarthritis: A systematic review. J. Rheumatol. 2020, 47, 748–760. [Google Scholar] [CrossRef]
- Macêdo, M.B.; Santos, V.M.O.S.; Pereira, R.M.R.; Fuller, R. Association between osteoarthritis and atherosclerosis: A systematic review and meta-analysis. Exp. Gerontol. 2022, 161, 111734. [Google Scholar] [CrossRef]
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, T.; Masaki, N.; Takase, B.; Adachi, T. Arterial stiffness assessed by cardio-ankle vascular index. Int. J. Mol. Sci. 2019, 20, 3664. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, K.; Ding, N.; Kim, E.D.; Budoff, M.; Chirinos, J.A.; Fernhall, B.; Hamburg, N.M.; Kario, K.; Miyoshi, T.; Tanaka, H.; et al. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens. 2019, 21, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Goh, G.S.; Liow, M.H.; Bin Abd Razak, H.R.; Tay, D.K.; Lo, N.N.; Yeo, S.J. Patient-reported outcomes, quality of life, and satisfaction rates in young patients aged 50 years or younger after total knee arthroplasty. J. Arthroplast. 2017, 32, 419–425. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.W.; Jang, Y.S. Long-term (up to 27 Years) prospective, randomized study of mobile-bearing and fixed-bearing total knee arthroplasties in patients < 60 years of age with osteoarthritis. J. Arthroplast. 2021, 36, 1330–1335. [Google Scholar]
- Kremers, H.M.; Sierra, R.J.; Schleck, C.D.; Berry, D.J.; Cabanela, M.E.; Hanssen, A.D.; Pagnano, M.W.; Trousdale, R.T.; Lewallen, D.G. Comparative survivorship of different tibial designs in primary total knee arthroplasty. J. Bone Jt. Surg. Am. 2014, 96, e121. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, V.; Conteduca, F.; Iorio, R.; Di Stasio, E.; Mazza, D.; Drogo, P.; Annibaldi, A.; Ferretti, A. Comorbidities rather than age affect medium-term outcome in octogenarian patients after total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, Y.A.; Al-Shahrani, F.S.; Alanazi, S.S.; Alshammari, F.A.; Alkhudair, A.M.; Jatoi, N.A. The Association of blood glucose levels and arterial stiffness (Cardio-ankle vascular index) in patients with type 2 diabetes mellitus. Cureus 2021, 13, e20408. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, D.; Imamura, H.; Sato, Y.; Yamaguchi, T.; Ban, N.; Kawana, H.; Nagumo, A.; Shirai, K.; Tatsuno, I. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: A cross-sectional study. Vasc. Health Risk Manag. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovska, I.; Kunzova, S.; Jakubik, J.; Hruskova, J.; Skladana, M.; Rivas-Serna, I.M.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Vysoky, R.; Geda, Y.E.; et al. Associations between high triglycerides and arterial stiffness in a population-based sample: Kardiovize Brno 2030 study. Lipids Health Dis. 2020, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Dobsak, P.; Soska, V.; Sochor, O.; Jarkovsky, J.; Novakova, M.; Homolka, M.; Soucek, M.; Palanova, P.; Lopez-Jimenez, F.; Shirai, K. Increased cardio-ankle vascular index in hyper-lipidemic patients without diabetes or hypertension. J. Atheroscler. Thromb. 2015, 22, 272–283. [Google Scholar] [CrossRef]
- Kubozono, T.; Miyata, M.; Ueyama, K.; Hamasaki, S.; Kusano, K.; Kubozono, O.; Tei, C. Acute and chronic effects of smoking on arterial stiffness. Circ. J. 2011, 75, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. American Society of Anaesthesiologists Physical Status Classification System. Available online: http://www.asahq.org/resources/clinical-information/asa-physical-statusclassification-system (accessed on 24 March 2023).
- Kellgren, J.H.; Lawrence, J.S. Radiographical assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Alicea, J. Scoring systems and their validation for the arthritic knee. In Surgery of the Knee, 3rd ed.; Insall, J.N., Scott, W.N., Eds.; Churchill Livingstone: New York, NY, USA, 2001; Volume 2, pp. 1507–1515. [Google Scholar]
- Shirai, K.; Hiruta, N.; Song, M.; Kurosu, T.; Suzuki, J.; Tomaru, T.; Miyashita, Y.; Saiki, A.; Takahashi, M.; Suzuki, K.; et al. Cardioankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J. Atheroscler. Thromb. 2011, 18, 924–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Chung, G.E.; Lee, H.; Kim, M.J.; Choi, S.Y.; Lee, W.; Yoon, J.W. Significance of Low Muscle mass on arterial stiffness as measured by cardio-ankle vascular index. Front. Cardiovasc. Med. 2022, 9, 857871. [Google Scholar] [CrossRef] [PubMed]
- Bovonratwet, P.; Fu, M.C.; Tyagi, V.; Gu, A.; Sculco, P.K.; Grauer, J.N. Is discharge within a day total knee arthroplasty safe in the octogenarian population? J. Arthroplast. 2019, 34, 235–241. [Google Scholar] [CrossRef]
- Hawker, G.A.; Croxford, R.; Bierman, A.S.; Harvey, P.J.; Ravi, B.; Stanaitis, I.; Lipscombe, L.L. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: A population based cohort study. PLoS ONE 2014, 9, e91286. [Google Scholar] [CrossRef]
- Uurtuya, S.; Taniguchi, N.; Kotani, K.; Yamada, T.; Kawano, M.; Khurelbaatar, N.; Itoh, K.; Lkhagvasuren, T. Comparative study of the cardio-ankle vascular index and ankle–brachial index between young Japanese and Mongolian subjects. Hypertens. Res. 2009, 32, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Yingchoncharoen, T.; Sritara, P. Cardio-ankle vascular index in a Thai population. Pulse 2017, 4 (Suppl. S1), 8–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shirai, K.; Liu, J.; Lu, N.; Wang, M.; Zhao, H.; Xie, J.; Yu, X.; Fu, X.; Shi, H.; et al. Comparative study of cardio-ankle vascular index between Chinese and Japanese healthy subjects. Clin. Exp. Hypertens. 2014, 36, 596–601. [Google Scholar] [CrossRef]



| Variables (Patients/Knees) | 209/251 |
|---|---|
| Sex (male vs. female) | 42/209 |
| Body height (cm) | 150 (146, 155) |
| Body weight (kg) | 59 (53, 67) |
| Body mass index (kg/m2) | 26 (24, 28) |
| Age (years) | 74 (69, 79), M; 76 (70, 81), F; 73 (69, 78) |
| Smoking history (yes/no) | 12/239 |
| Diabetes mellitus (yes/no) | 35/216 |
| Hypertension (yes/no) | 164/87 |
| Preop. blood cholesterol level (mg/dL) | 205 (185, 234) |
| Preop. blood triglyceride level (mg/dL) | 132 (101, 175) |
| Knee flexion (Preop) (°) | 115 (100, 125) |
| Knee extension (Preop) (°) | −10.0 (−15, −5) |
| Knee range of motion (Preop) (°) | 100 (90, 120) |
| HSS score [22] | 45 (37, 52) |
| Kellgren–Laurence classification [21] | I 0, II 0, III 10, IV 241 |
| ASA grade [20] | I 34, II 217 |
| Variables | r | p |
|---|---|---|
| Pre CAVI (contra-lateral) | 0.901 | p < 0.001 |
| Age | 0.451 | p < 0.001 |
| Body height | −0.049 | 0.442 |
| Body weight | −0.307 | p < 0.001 |
| Body mass index | −0.322 | p < 0.001 |
| HSS score [22] | −0.089 | p = 0.160 |
| Flexion | 0.069 | 0.275 |
| Extension | −0.096 | 0.130 |
| ROM | 0.024 | 0.700 |
| Cholesterol | 0.025 | 0.695 |
| Triglyceride | 0.044 | 0.484 |
| Variables Knees (Patients) | Median (Interquartile) Range | p | |
|---|---|---|---|
| Sex: male/female 42 (36) (17%)/209 (173) (83%) | Male; 9.1 (8.4, 9.7) 6.4–11.9 | Female; 8.8 (7.8, 9.6) 3.1–12.0 | 0.223 |
| KL [21]: III 10 (4%), IV 241 (96%) | III; 9.1 (8.0, 9.4) 6.9–10.6 | IV; 8.8 (8.0, 9.7) 3.1–12.0 | 0.950 |
| ASA [20]: I 34 (14%), II 217 (86%) | I; 8.9 (7.8, 9.9) 6.2–11.6 | II; 8.9 (8.0, 9.6) 3.1–12 | 0.691 |
| Smoking history: yes 12 (5%) | Yes; 8.8 (8.5, 9.3) 6.7–10.4 | No; 8.9 (8.0, 9.7) 3.1–12.0 | 0.987 |
| Hypertension: yes 164 (65%) | Yes; 9.0 (8.1, 9.7) 3.1–12.0 | No; 8.7 (7.7, 9.6) 6.2–11.7 | 0.078 |
| Diabetes mellitus: yes 35 (14%) | Yes; 8.9 (8.0, 9.5) 7.0–11.6 | No; 8.8 (8.0, 9.7) 3.1–12.0 | 0.962 |
| B | S.E. | β | Sig. | 95% CI | ||
|---|---|---|---|---|---|---|
| (Constant) | 6.699 | 0.920 | <0.001 | 4.887 | 8.512 | |
| Age | 0.055 | 0.009 | 0.349 | <0.001 | 0.035 | 0.073 |
| BMI | −0.072 | 0.015 | −0.235 | <0.001 | −0.107 | −0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, Y.; Noguchi, H.; Sato, J.; Takahashi, I.; Ishii, H.; Ishii, R.; Ishii, K.; Ishii, K.; Toyabe, S.-i. Characteristics of Preoperative Arteriosclerosis Evaluated by Cardio-Ankle Vascular Index in Patients with Osteoarthritis before Total Knee Arthroplasty. J. Clin. Med. 2023, 12, 4685. https://doi.org/10.3390/jcm12144685
Ishii Y, Noguchi H, Sato J, Takahashi I, Ishii H, Ishii R, Ishii K, Ishii K, Toyabe S-i. Characteristics of Preoperative Arteriosclerosis Evaluated by Cardio-Ankle Vascular Index in Patients with Osteoarthritis before Total Knee Arthroplasty. Journal of Clinical Medicine. 2023; 12(14):4685. https://doi.org/10.3390/jcm12144685
Chicago/Turabian StyleIshii, Yoshinori, Hideo Noguchi, Junko Sato, Ikuko Takahashi, Hana Ishii, Ryo Ishii, Kei Ishii, Kai Ishii, and Shin-ichi Toyabe. 2023. "Characteristics of Preoperative Arteriosclerosis Evaluated by Cardio-Ankle Vascular Index in Patients with Osteoarthritis before Total Knee Arthroplasty" Journal of Clinical Medicine 12, no. 14: 4685. https://doi.org/10.3390/jcm12144685