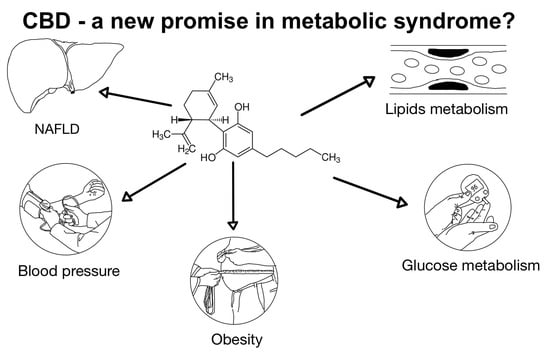
The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing?
Abstract
:1. Introduction
- (1)
- Increased waist circumference (depends on country of origin and ethnic group—in the European Caucasian population, ≥80 cm in women and ≥94 cm in men);
- (2)
- Fasting triglycerides > 1.7 mmol/L (150 mg/dL) or treatment for hypertriglyceridemia;
- (3)
- Fasting HDL-C < 1.0 mmol/L (40 mg/dL) in men and <1.3 mmol/L (50 mg/dL) in women or treatment for this lipid disorder;
- (4)
- Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, or treatment of previously diagnosed hypertension;
- (5)
- Fasting plasma glucose ≥ 5.6 mmol/L (100 mg/dL) or medication for type 2 diabetes.
2. The Endocannabinoid System—Biological Effect of Cannabinoids
| Selected Phytocannabinoids | Role | Receptors |
|---|---|---|
| Cannabidiol (CBD) | Agonist or Positive allosteric modulator | TRPA, TRPV1/2, α1-GlyR, GABAAR, A1AR/A2AR, and 5HT1AR/5HT2AR. |
| Antagonist or Inverse agonist or Negative allosteric modulator | 5HT3AR, Nav1.1–1.7, GPR55, GPR18, GPR3, GPR6, GPR12, Kv2.1, TRPM8, Cav3.1–3.3, α7nACh, δ-OR, and µ-OR. | |
| Δ9-Tetrahydrocannabinol (Δ9-THC) | Agonist or Positive allosteric modulator | GPR55, GPR18, PPAR-γ, TRPA1, TRPV2, 5-HT2AR, α1-GlyR, and α1β1-GlyR. |
| Antagonist or Inverse agonist or Negative allosteric modulator | 5HT3AR, δ-OR, and µ-OR TRPM8. |
3. Methodology
4. Cannabinoids Relationship with Obesity
5. Cannabinoids Impact on Glucose Metabolism
6. Plasma Lipid Level and Cannabis
| Authors | Characteristics of the Study Group | Cannabis Formulation Characteristics | Statistically Significant Changes in Lipid Metabolism Parameters | Duration of the Observation | Reference |
|---|---|---|---|---|---|
| Zanoni et. al | Human hepatic HepG2 cells, in vitro study. | Ninety different peptides are derived from Cannabis sativa hydrolysis. | Inhibition of HMG-CoAR activity ↑LDLR expression ↑PCSK9. | - | [79] |
| Afshar et al. | DM2, mean age 55.7 years old. | Sublingual spray CBDEX10 twice daily (200 µg/20 µg CBD/Δ9-THC). | ↓TC, ↓TG, and ↓LDL. | 8 weeks. | [92] |
| Abuhasira et al. | HT, PD, and mean age 69 years old. | Herbal cannabis supplements with different CBD:THC ratios and different dosing patterns. | Positive correlation of 2-AG and TG change and negative correlation of HDL/LDL ratio and OEA and PEA. | 3 months. | [93] |
| Cusihuaman et al. | Mean age 31 years old, otherwise healthy, declared daily-smokers of marijuana for at least 12 months | Approximately 0.2 g of Cannabis spp. administration by the pyrolytic route. | ↑HDL at 120 min. | Measurements at 30 and 120 min after smoking. | [87] |
| Li et al. | human hepatic HepG2 cells: an in vitro study. | Cannabis sativa Peptide H3 (IGFLIIWV) | Inhibition of HMGCoAR activity, ↑LDLR expression, and ↓PCSK9. | - | [80] |
| Huang et al. | Animal model of atherosclerosis: apoE-deficient mice fed with a high-cholesterol diet. | cannabis seed oil; 30 μL by gavage using a micropipettor per day. (7.4% of palmitic acid, 3.0% of stearic acid, 10.8% of oleic acid, 55.8% of linoleic acid, 14.0% of α-linolenic acid, and 2.5% of γ-linolenic acid). | ↓TG ↓LDL at week 6. | Measurements at weeks 4, 6, and 8. | [84] |
| Abbotts et al. | Mean age 26 years old, mean BMI 29.7. | Five different formulations, each containing 30 mg of CBD; seven doses (2 followed by a meal, five unrelated to the meal). | ↓postprandial TG at 30 min. | Series of measures up to 240 min after CBD ingestion. | [76] |
| Alonso et al. | Schizophrenia. | Declared daily cannabis use. | Positive correlation between cannabis use and HDL. | One year follow-up. | [89] |
| Reyes-Cuapio et al. | Juvenile Wistar rats at 30 postanal day | CBD intraperitoneal injections (5, 10, or 30 mg/kg). | ↓TG | 14 days. | [82] |
| Kaushal et al. | animal model of induced hypercholesterolemia. Wistar rats fed with high-fat diet. | Special composition of hempseed diet for 1 or 2 months. | ↓TG, ↑HDL, ↓LDL, and ↓TC. | 1 or 2 months. | [83] |
| Ben-Cnaan et al. | Mice models of diet and genetic induced obesity. | CBDA-O-methyl ester (HU-580, EPM301) 40 mg/kg/day, intraperitoneal administration. | ↓TG, ↓TC, and ↑HDL/LDL. | 28 days. | [86] |
| Stiles et al. | Mean age: 23.4 years old, first episode of psychosis. | Analysis of data obtained from RAISE-ETP study. | ↓TG. | 24 months. | [91] |
7. Cannabinoids Effects on Blood Pressure
8. Non-Alcoholic Fatty Liver Disease and Its Correlation with Cannabis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNODC. World Drug Report 2022 Highlights Trends on Cannabis Post-Legalization, Environmental Impacts of Illicit Drugs, and Drug Use among Women and Youth; UNODC: Vienna, Austria, 2023. [Google Scholar]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S163. [Google Scholar] [CrossRef] [PubMed]
- Golombek, P.; Müller, M.; Barthlott, I.; Sproll, C.; Lachenmeier, D.W. Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature. Toxics 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Zuardi, A.W.; Alexandre, S.; Crippa, J. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Janecki, M.; Graczyk, M.; Lewandowska, A.A.; Pawlak, Ł. Anti-Inflammatory and Antiviral Effects of Cannabinoids in Inhibiting and Preventing SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 4170. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177. [Google Scholar] [CrossRef]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Gilman, J.M.; Schuster, R.M.; Potter, K.W.; Schmitt, W.; Wheeler, G.; Pachas, G.N.; Hickey, S.; Cooke, M.E.; Dechert, A.; Plummer, R.; et al. Effect of Medical Marijuana Card Ownership on Pain, Insomnia, and Affective Disorder Symptoms in Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e222106. [Google Scholar] [CrossRef]
- Kuharic, D.B.; Markovic, D.; Brkovic, T.; Kegalj, M.J.; Rubic, Z.; Vukasovic, A.V.; Jeroncic, A.; Puljak, L. Cannabinoids for the treatment of dementia. Cochrane Database Syst. Rev. 2021. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.-J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective Effect of(−)Δ9-Tetrahydrocannabinol and Cannabidiol in N-Methyl-d-Aspartate-Induced Retinal Neurotoxicity: Involvement of Peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef]
- Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasingam, S.J.; Ranjith, B.; Jackson, R.; Wijeratne, D.T. Cannabis and its derivatives for the use of motor symptoms in Parkinson’s disease: A systematic review and meta-analysis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211018561. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and Epilepsy. Neurotherapeutics 2015, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018, 2020, CD012182. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Olinga, P.; Kok, E.A.; Brugman, D.A.P.; van Ark, U.F.; Ramcharan, A.S.; Lebbink, P.W.; Hoogwater, F.J.H.; Knapen, D.G.; de Groot, D.J.A.; et al. Potential, Limitations and Risks of Cannabis-Derived Products in Cancer Treatment. Cancers 2023, 15, 2119. [Google Scholar] [CrossRef]
- Barré, T.; Bourlière, M.; Ramier, C.; Carrat, F.; Di Beo, V.; Protopopescu, C.; Marcellin, F.; Bureau, M.; Cagnot, C.; Dorival, C.; et al. Cannabis Use Is Inversely Associated with Metabolic Disorders in Hepatitis C-Infected Patients (ANRS CO22 Hepather Cohort). J. Clin. Med. 2022, 11, 6135. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Hollman, G.; Kristenson, M. The prevalence of the metabolic syndrome and its risk factors in a middle-aged Swedish population—Mainly a function of overweight? Eur. J. Cardiovasc. Nurs. 2008, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Desroches, S.; Lamarche, B. The evolving definitions and increasing prevalence of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, P.; Prejbisz, A.; Kuryłowicz, A.; Baska, A.; Burchardt, P.; Chlebus, K.; Dzida, G.; Jankowski, P.; Jaroszewicz, J.; Jaworski, P.; et al. Metabolic syndrome—A new definition and management guidelines: A joint position paper by the Polish Society of Hypertension, Polish Society for the Treatment of Obesity, Polish Lipid Association, Polish Association for Study of Liver, Polish Society of Family Medicine, Polish Society of Lifestyle Medicine, Division of Prevention and Epidemiology Polish Cardiac Society, “Club 30” Polish Cardiac Society, and Division of Metabolic and Bariatric Surgery Society of Polish Surgeons. Arch. Med. Sci. 2022, 18, 1133. [Google Scholar] [CrossRef] [PubMed]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Busquets Garcia, A.; Soria-Gomez, E.; Bellocchio, L.; Marsicano, G. Cannabinoid receptor type-1: Breaking the dogmas. F1000Research 2016, 5, 990. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzien, M.; Pawinski, T.; Sulkowska, A.J.I. A Guide to Targeting the Endocannabinoid System in Drug Design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Mastinu, A.; Premoli, M.; Ferrari-Toninelli, G.; Tambaro, S.; Maccarinelli, G.; Memo, M.; Bonini, S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20180013. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Castorena, C.M.; Vianna, C.R.; Lee, C.E.; Metzger, N.P.; Vijayaraghavan, P.; Osborne-Lawrence, S.; Elmquist, J.K.; Zigman, J.M. Combined Loss of Ghrelin Receptor and Cannabinoid CB1 Receptor in Mice Decreases Survival but does not Additively Reduce Body Weight or Eating. Neuroscience 2019, 447, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of cannabinoids in obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef]
- Tam, J.; Cinar, R.; Liu, J.; Godlewski, G.; Wesley, D.; Jourdan, T.; Szanda, G.; Mukhopadhyay, B.; Chedester, L.; Liow, J.-S.; et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012, 16, 167–179. [Google Scholar] [CrossRef]
- Malcher-Lopes, R.; Di, S.; Marcheselli, V.S.; Weng, F.J.; Stuart, C.T.; Bazan, N.G.; Tasker, J.G. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J. Neurosci. 2006, 26, 6643–6650. [Google Scholar] [CrossRef]
- Dörnyei, G.; Vass, Z.; Juhász, C.B.; Nádasy, G.L.; Hunyady, L.; Szekeres, M. Role of the Endocannabinoid System in Metabolic Control Processes and in the Pathogenesis of Metabolic Syndrome: An Update. Biomedicines 2023, 11, 306. [Google Scholar] [CrossRef]
- Janero, D.R. Cannabinoid-1 receptor (CB1R) blockers as medicines: Beyond obesity and cardiometabolic disorders to substance abuse/drug addiction with CB1R neutral antagonists. Expert Opin. Emerg. Drugs 2012, 17, 17–29. [Google Scholar] [CrossRef]
- Frieling, H.; Albrecht, H.; Jedtberg, S.; Gozner, A.; Lenz, B.; Wilhelm, J.; Hillemacher, T.; de Zwaan, M.; Kornhuber, J.; Bleich, S. Elevated cannabinoid 1 receptor mRNA is linked to eating disorder related behavior and attitudes in females with eating disorders. Psychoneuroendocrinology 2009, 34, 620–624. [Google Scholar] [CrossRef]
- Hirsch, S.; Tam, J. Cannabis: From a Plant That Modulates Feeding Behaviors toward Developing Selective Inhibitors of the Peripheral Endocannabinoid System for the Treatment of Obesity and Metabolic Syndrome. Toxins 2019, 11, 275. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Berk, K.; Bzdega, W.; Kolakowski, A.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Endocannabinoid System and Physical Activity—A Robust Duo in the Novel Therapeutic Approach against Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3083. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: D 9-tetrahydrocannabinol, cannabidiol and D 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef]
- Fearby, N.; Penman, S.; Thanos, P. Effects of D9-Tetrahydrocannibinol (THC) on Obesity at Different Stages of Life: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 3174. [Google Scholar] [CrossRef]
- Grimison, P.; Mersiades, A.; Kirby, A.; Lintzeris, N.; Morton, R.; Haber, P.; Olver, I.; Walsh, A.; McGregor, I.; Chenung, Y.; et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann. Oncol. 2020, 31, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- French, J.A.; Krauss, G.L.; Wechsler, R.T.; Wang, X.-F.; DiVentura, B.; Brandt, C.; Trinka, E.; O’Brien, T.J.; Laurenzam, A.; Patten, A.; et al. Randomized, doseranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef]
- Miller, I.; Scheffer, I.E.; Gunning, B.; Sanchez-Carpintero, R.; Gil-Nagel, A.; Perry, M.S.; Saneto, R.P.; Checketts, D.; Dunayevich, E.; Knappertz, V. Dose-ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: A randomized clinical trial. JAMA Neurol. 2020, 77, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Harel, M.; Cassuto, H.; Polyansky, L.; Schnapp, A.; Wattad, N.; Shmueli, D.; Golan, D.; Castellanos, F.X. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol. Autism 2021, 12, 6. [Google Scholar] [CrossRef]
- Palrasu, M.; Wright, L.; Patel, M.; Leech, L.; Branch, S.; Harrelson, S.; Khan, S. Perspectives on Challenges in Cannabis Drug Delivery Systems: Where Are We? Med. Cannabis Cannabinoids 2022, 5, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Mboumba Bouassa, R.-S.; Sebastiani, G.; Di Marzo, V.; Jenabian, M.-A.; Costiniuk, C.T. Cannabinoids and Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 9423. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Foltin, R.W.; Fischman, M.W.; Byrne, M.F. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 1988, 11, 1–14. [Google Scholar] [CrossRef]
- Clark, T.M.; Jones, J.M.; Hall, A.G.; Tabner, S.A.; Kmiec, R.L. Theoretical Explanation for Reduced Body Mass Index and Obesity Rates in Cannabis Users. Cannabis Cannabinoid Res. 2018, 3, 259. [Google Scholar] [CrossRef] [PubMed]
- Le Strat, Y.; Le Foll, B. Obesity and cannabis use: Results from 2 representative national surveys. Am. J. Epidemiol. 2011, 174, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Ignatowska-Jankowska, B.; Jankowski, M.M.; Swiergiel, A.H. Cannabidiol decreases body weight gain in rats: Involvement of CB2 receptors. Neurosci. Lett. 2011, 490, 82–84. [Google Scholar] [CrossRef]
- Mathes, C.M.; Ferrara, M.; Rowland, N.E. Selection of a palatable dietary option is not preferentially reduced by cannabinoid CB1 receptor antagonist AM251 in female C57Bl/6J mice. Pharmacol. Biochem. Behav. 2009, 94, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wierucka-Rybak, M.; Wolak, M.; Bojanowska, E. The effects of leptin in combination with a cannabinoid receptor 1 antagonist, AM 251, or cannabidiol on food intake and body weight in rats fed a high-fat or a free-choice high sugar diet. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2014, 65, 487–496. [Google Scholar]
- Bielawiec, P.; Harasim-Symbor, E.; Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Chabowski, A. Attenuation of oxidative stress and inflammatory response by chronic cannabidiol administration is associated with improved n-6/n-3 pufa ratio in the white and red skeletal muscle in a rat model of high-fat diet-induced obesity. Nutrients 2021, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, S.E.M.; de Graaf, C.; Witkamp, R.F.; Jager, G. Explorative Placebo-Controlled Double-Blind Intervention Study with Low Doses of Inhaled Δ9-Tetrahydrocannabinol and Cannabidiol Reveals No Effect on Sweet Taste Intensity Perception and Liking in Humans. Cannabis Cannabinoid Res. 2017, 2, 114–122. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Pacheco, J.C.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.D.L.; Loureiro, S.R.; dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. 2022, 7, 658–669. [Google Scholar] [CrossRef]
- Aseer, K.R.; Egan, J.M. An Autonomous Cannabinoid System in Islets of Langerhans. Front. Endocrinol. 2021, 12, 699661. [Google Scholar] [CrossRef]
- Gatta-Cherifi, B.; Cota, D. New insights on the role of the endocannabinoid system in the regulation of energy balance. Int. J. Obes. 2016, 40, 210–219. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Olofinsan, K.A.; Oyedemi, S.O.; Chukwuma, C.I.; Nde, A.L.; Islam, S. Cannabidiol improves glucose utilization and modulates glucose-induced dysmetabolic activities in isolated rats’ peripheral adipose tissues. Biomed. Pharmacother. 2022, 149, 112863. [Google Scholar] [CrossRef]
- Cinar, R.; Godlewski, G.; Liu, J.; Tam, J.; Jourdan, T.; Mukhopadhyay, B.; Harvey-White, J.; Kunos, G. Hepatic CB1 Receptors Mediate Diet-Induced Insulin Resistance by Increasing de novo Synthesis of Long Chain Ceramides. Hepatology 2014, 59, 143–153. [Google Scholar] [CrossRef]
- Berk, K.; Konstantynowicz-Nowicka, K.; Charytoniuk, T.; Harasim-Symbor, E.; Chabowski, A. Distinct Effects of Cannabidiol on Sphingolipid Metabolism in Subcutaneous and Visceral Adipose Tissues Derived from High-Fat-Diet-Fed Male Wistar Rats. Int. J. Mol. Sci. 2022, 23, 5382. [Google Scholar] [CrossRef] [PubMed]
- Bielawiec, P.; Harasim-Symbor, E.; Konstantynowicz-Nowicka, K.; Sztolsztener, K.; Chabowski, A. Chronic Cannabidiol Administration Attenuates Skeletal Muscle De Novo Ceramide Synthesis Pathway and Related Metabolic Effects in a Rat Model of High-Fat Diet-Induced Obesity. Biomolecules 2020, 10, 1241. [Google Scholar] [CrossRef]
- Gorelick, J.; Assa-Glazer, T.; Gil Zandani, G.; Altberg, A.; Sela, N.; Nyska, A.; Madar, Z. THC and CBD affect metabolic syndrome parameters including microbiome in mice fed high fat-cholesterol diet. J. Cannabis Res. 2022, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Patrician, A.; Versic-Bratincevic, M.; Mijacika, T.; Banic, I.; Marendic, M.; Sutlović, D.; Dujić, Ž.; Ainslie, P.N. Examination of a New Delivery Approach for Oral Cannabidiol in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Pharmacokinetics Study. Adv. Ther. 2019, 36, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Abbotts, K.S.S.; Ewell, T.R.; Butterklee, H.M.; Bomar, M.C.; Akagi, N.; Dooley, G.P.; Bell, C. Cannabidiol and Cannabidiol Metabolites: Pharmacokinetics, Interaction with Food, and Influence on Liver Function. Nutrients 2022, 14, 2152. [Google Scholar] [CrossRef]
- Moore, B.F.; A Sauder, K.; Shapiro, A.L.B.; Crume, T.; Kinney, G.L.; Dabelea, D. Fetal Exposure to Cannabis and Childhood Metabolic Outcomes: The Healthy Start Study. J. Clin. Endocrinol. Metab. 2022, 107, e2862. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef]
- Li, J.; Bollati, C.; Bartolomei, M.; Mazzolari, A.; Arnoldi, A.; Vistoli, G.; Lammi, C. Hempseed (Cannabis sativa) Peptide H3 (IGFLIIWV) Exerts Cholesterol-Lowering Effects in Human Hepatic Cell Line. Nutrients 2022, 14, 1804. [Google Scholar] [CrossRef]
- Morales, P.; Jagerovic, N. Synthetic and Natural Derivatives of Cannabidiol. Adv. Exp. Med. Biol. 2021, 1297, 11–25. [Google Scholar] [CrossRef]
- Reyes-Cuapio, E.; Coronado-Álvarez, A.; Quiroga, C.; Alcaraz-Silva, J.; Ruíz-Ruíz, J.C.; Imperatori, C.; Murillo-Rodríguez, E. Juvenile cannabidiol chronic treatments produce robust changes in metabolic markers in adult male Wistar rats. Eur. J. Pharmacol. 2021, 910, 174463. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zeng, Z.; Lang, Y.; Xiang, X.; Qi, G.; Lu, G.; Yang, X. Cannabis Seed Oil Alleviates Experimental Atherosclerosis by Ameliorating Vascular Inflammation in Apolipoprotein-E-Deficient Mice. J. Agric. Food Chem. 2021, 69, 9102–9110. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Hotamisligil, G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021, 3, 1302–1312. [Google Scholar] [CrossRef]
- Ben-Cnaan, E.; Permyakova, A.; Azar, S.; Hirsch, S.; Baraghithy, S.; Hinden, L.; Tam, J. The Metabolic Efficacy of a Cannabidiolic Acid (CBDA) Derivative in Treating Diet- and Genetic-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 5610. [Google Scholar] [CrossRef] [PubMed]
- Cusihuaman, S.; Moya-Salazar, J.; Wong-Salgado, P.; Moya-Salazar, M.M.; Cañari, B.; Chicoma-Flores, K.; Contreras-Pulache, H. Changes in High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL) and Cholesterol Concentration in Heavy Cannabis Users: A Single-Centre Study in Cusco, Peru. Processes 2022, 10, 1597. [Google Scholar] [CrossRef]
- Lazarte, J.; Hegele, R.A. Cannabis effects on lipoproteins. Curr. Opin. Lipidol. 2019, 30, 140–146. [Google Scholar] [CrossRef]
- Alonso, Y.; Miralles, C.; Algora, M.J.; Valiente-Pallejà, A.; Sánchez-Gistau, V.; Muntané, G.; Labad, J.; Vilella, E.; Martorell, L. Risk factors for metabolic syndrome in individuals with recent-onset psychosis at disease onset and after 1-year follow-up. Sci. Rep. 2022, 12, 11386. [Google Scholar] [CrossRef]
- Scheffler, F.; Kilian, S.; Chiliza, B.; Asmal, L.; Phahladira, L.; du Plessis, S.; Kidd, M.; Murray, R.; Di Forti, M.; Seedat, S.; et al. Effects of cannabis use on body mass, fasting glucose and lipids during the first 12 months of treatment in schizophrenia spectrum disorders. Schizophr. Res. 2018, 199, 90–95. [Google Scholar] [CrossRef]
- Stiles, E.; Alcover, K.C.; Stiles, B.; Oluwoye, O.; McDonell, M.G. Cannabis use and metabolic syndrome among clients with first episode psychosis. Early Interv. Psychiatry 2021, 15, 1051–1055. [Google Scholar] [CrossRef]
- Afshar, S.; Khalili, S.; Amin, G.; Abbasinazari, M. A Phase I Randomized, Double-blind, Placebo-controlled Study on Efficacy and Safety Profile of a Sublingually Administered Cannabidiol /Delta 9-tetrahydrocannabidiol (10: 1) Regimen in Diabetes Type 2 Patients. Iran. J. Pharm. Res. 2023, 21, e132647. [Google Scholar] [CrossRef] [PubMed]
- Abuhasira, R.; Azar, S.; Nemirovski, A.; Tam, J.; Novack, V. Herbal Cannabis Use Is Not Associated with Changes in Levels of Endocannabinoids and Metabolic Profile Alterations among Older Adults. Life 2022, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, F.; Mezzullo, M.; Belluomo, I.; Di Lallo, V.D.; Baccini, M.; Gasparini, D.I.; Casadio, E.; Mastroroberto, M.; Vicennati, V.; Gambineri, A.; et al. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol. Metab. 2017, 6, 406–415. [Google Scholar] [CrossRef]
- Al Ghorani, H.; Götzinger, F.; Böhm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. [Google Scholar] [CrossRef]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Batinic, A.; Sutlović, D.; Kuret, S.; Matana, A.; Kumric, M.; Bozic, J.; Dujic, Z. Trial of a Novel Oral Cannabinoid Formulation in Patients with Hypertension: A Double-Blind, Placebo-Controlled Pharmacogenetic Study. Pharmaceuticals 2023, 16, 645. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Gryczka, K.; Kurant, D.; Szambelan, M.; Malinowski, B.; Falkowski, M.; Zabrzyński, J.; Słupski, M. The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing? J. Clin. Med. 2023, 12, 4620. https://doi.org/10.3390/jcm12144620
Wiciński M, Fajkiel-Madajczyk A, Kurant Z, Gryczka K, Kurant D, Szambelan M, Malinowski B, Falkowski M, Zabrzyński J, Słupski M. The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing? Journal of Clinical Medicine. 2023; 12(14):4620. https://doi.org/10.3390/jcm12144620
Chicago/Turabian StyleWiciński, Michał, Anna Fajkiel-Madajczyk, Zuzanna Kurant, Karol Gryczka, Dominik Kurant, Monika Szambelan, Bartosz Malinowski, Michal Falkowski, Jan Zabrzyński, and Maciej Słupski. 2023. "The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing?" Journal of Clinical Medicine 12, no. 14: 4620. https://doi.org/10.3390/jcm12144620