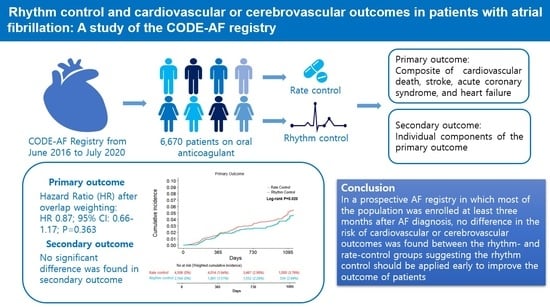
Rhythm Control and Cardiovascular or Cerebrovascular Outcomes in Patients with Atrial Fibrillation: A Study of the CODE-AF Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Rate and Rhythm Control Strategies
2.3. Outcome Definition and Follow-Up
2.4. Statistical Analysis
2.5. Subgroup Analysis
3. Results
3.1. Study Population
3.2. Clinical Outcomes
3.3. Subgroup Analysis
4. Discussion
4.1. The Timing of Rhythm Control Initiation
4.2. The Study Population
4.3. Rhythm Control Methods
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Camm, A.J.; Accetta, G.; Ambrosio, G.; Atar, D.; Bassand, J.-P.; Berge, E.; Cools, F.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017, 103, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Marijon, E.; Le Heuzey, J.-Y.; Connolly, S.; Yang, S.; Pogue, J.; Brueckmann, M.; Eikelboom, J.; Themeles, E.; Ezekowitz, M.; Wallentin, L.; et al. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013, 128, 2192–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, D.B.; Anstrom, K.J.; Sheng, S.; Piccini, J.P.; Baloch, K.N.; Monahan, K.H.; Daniels, M.R.; Bahnson, T.D.; Poole, J.E.; Rosenberg, Y.; et al. Effect of Catheter Ablation vs. Medical Therapy on Quality of Life among Patients with Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019, 321, 1275–1285. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.; Tijssen, J.G.; et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, J.; Miketic, S.; Windeler, J.; Cuneo, A.; Haun, S.; Micus, S.; Walter, S.; Tebbe, U. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: The Strategies of Treatment of Atrial Fibrillation (STAF) study. J. Am. Coll. Cardiol. 2003, 41, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; You, S.C.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: Nationwide cohort study. BMJ 2021, 373, n991. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; You, S.C.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.; Sung, J.-H.; et al. Age and Outcomes of Early Rhythm Control in Patients with Atrial Fibrillation: Nationwide Cohort Study. JACC Clin. Electrophysiol. 2022, 8, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.-S.; You, S.C.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.; Sung, J.-H.; et al. Early Rhythm Control Therapy for Atrial Fibrillation in Low-Risk Patients: A Nationwide Propensity Score-Weighted Study. Ann. Intern. Med. 2022, 175, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.-S.; You, S.C.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; et al. Association of rhythm control with incident dementia among patients with atrial fibrillation: A nationwide population-based cohort study. Age Ageing 2022, 51, afab248. [Google Scholar] [CrossRef]
- Yang, E.; Tang, O.; Metkus, T.; Berger, R.D.; Spragg, D.D.; Calkins, H.G.; Marine, J.E. The role of timing in treatment of atrial fibrillation: An AFFIRM substudy. Heart Rhythm. 2021, 18, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, T.-H.; Cha, M.-J.; Lee, J.M.; Park, J.; Park, J.-K.; Kang, K.-W.; Shim, J.; Uhm, J.-S.; Kim, J.; et al. A Prospective Survey of Atrial Fibrillation Management for Real-world Guideline Adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ. J. 2017, 47, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.S.; Kim, J.; Lee, Y.S.; Kim, T.-H.; Lee, J.M.; Park, J.; Park, J.-K.; Kang, K.-W.; Shim, J.; Uhm, J.-S.; et al. Current Anticoagulant Usage Patterns and Determinants in Korean Patients with Nonvalvular Atrial Fibrillation. Yonsei Med. J. 2020, 61, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kang, K.-W.; Kim, T.-H.; Cha, M.-J.; Lee, J.-M.; Park, J.; Park, J.-K.; Shim, J.; Uhm, J.-S.; Kim, J.; et al. Comparison of Rhythm and Rate Control Strategies for Stroke Occurrence in a Prospective Cohort of Atrial Fibrillation Patients. Yonsei Med. J. 2018, 59, 258–264. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Kim, J.-B.; Park, J.; Park, J.-K.; Kang, K.-W.; Shim, J.; Choi, E.-K.; Lee, Y.S.; Park, H.W.; et al. Association of Gender with Clinical Outcomes in a Contemporary Cohort of Patients with Atrial Fibrillation Receiving Oral Anticoagulants. Korean Circ. J. 2022, 52, 593–603. [Google Scholar] [CrossRef]
- Brinkmann, C.; Schofer, J. 4th universal definition of myocardial infarction 2018: What is new? Herz 2018, 43, 681–688. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Marinelli, E.A.; Arbelo, E.; Boriani, G.; Boveda, S.; Buckley, C.M.; Camm, A.J.; Casadei, B.; Chua, W.; Dagres, N.; et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: The 8th AFNET/EHRA consensus conference. Europace 2023, 25, 6–27. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305315. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wazni, O.M.; Kuniss, M.; Hawkins, N.M.; Deyell, M.W.; Chierchia, G.-B.; Nissen, S.; Verma, A.; Wells, G.A.; Turgeon, R.D. Cryoballoon Ablation as Initial Treatment for Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lane, D.A.; Zhang, H.; Wang, H.; Zhang, W.; Wen, J.; Xing, Y.; Wu, F.; Xia, Y.; Liu, T.; et al. Mobile Health Technology to Improve Care for Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 1523–1534. [Google Scholar] [CrossRef]
- Freedman, B.; Camm, J.; Calkins, H.; Healey, J.S.; Rosenqvist, M.; Wang, J.; Albert, C.M.; Anderson, C.S.; Antoniou, S.; Benjamin, E.J.; et al. Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation 2017, 135, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Chen, L.Y.; et al. Atrial Fibrillation and Dementia: A Report from the AF-SCREEN International Collaboration. Circulation 2022, 145, 392–409. [Google Scholar] [CrossRef]
- Svennberg, E.; Friberg, L.; Frykman, V.; Al-Khalili, F.; Engdahl, J.; Rosenqvist, M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): A multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021, 398, 1498–1506. [Google Scholar] [CrossRef]
- Rillig, A.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.J.; Goette, A.; Kuck, K.-H.; Metzner, A.; Vardas, P.; Vettorazzi, E.; et al. Early Rhythm Control in Patients with Atrial Fibrillation and High Comorbidity Burden. Circulation 2022, 146, 836–847. [Google Scholar] [CrossRef]
- Rillig, A.; Magnussen, C.; Ozga, A.-K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients with Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef]



| Before Overlap Weighting | After Overlap Weighting | |||||
|---|---|---|---|---|---|---|
| Rate Control (N = 4506) | Rhythm Control (N = 2164) | ASD | Rate Control (N = 1293 [4506] *) | Rhythm Control (N = 1293 [2164] *) | ASD | |
| Age in years | 70.1 (9.7) | 66.0 (12.1) | 0.379 | 67.6 (10.3) | 67.6 (12.8) | <0.001 |
| Male | 2712 (60.2) | 1440 (66.5) | 0.132 | 829 (64.1) | 829 (64.1) | <0.001 |
| Body mass index, kg/m2 | 24.8 (3.5) | 24.9 (3.2) | 0.032 | 24.9 (3.4) | 24.9 (3.2) | <0.001 |
| Systolic BP, mmHg | 122.3 (15.8) | 124.7 (15.4) | 0.156 | 124.0 (16.0) | 124.0 (15.3) | <0.001 |
| Diastolic BP, mmHg | 74.3 (12.0) | 75.6 (17.1) | 0.090 | 74.9 (12.0) | 74.9 (11.7) | <0.001 |
| Heart rate, beats/min | 77.4 (21.4) | 75.6 (17.1) | 0.091 | 75.9 (18.1) | 75.9 (17.5) | <0.001 |
| Type of AF | 0.228 | <0.001 | ||||
| Paroxysmal | 2649 (58.8) | 1319 (61.0) | 796 (61.5) | 796 (61.5) | ||
| Persistent | 1634 (36.3) | 822 (38.0) | 480 (37.1) | 480 (37.1) | ||
| Permanent | 222 (4.9) | 23 (1.1) | 18 (1.4) | 18 (1.4) | ||
| Onset of AF | 0.052 | <0.001 | ||||
| <3 month | 830 (18.4) | 433 (20.0) | 253 (19.6) | 253 (19.6) | ||
| ≥3 month | 3676 (81.6) | 1731 (80.0) | 1040 (80.4) | 1040 (80.4) | ||
| Alcohol intake ** | 1197 (26.6) | 646 (29.8) | 0.082 | 371 (28.7) | 371 (28.7) | <0.001 |
| Current smoking | 1270 (28.2) | 716 (33.1) | 0.107 | 402 (31.0) | 401 (31.0) | <0.001 |
| CHA2DS2-VASc score | 3.2 (1.6) | 2.6 (1.6) | 0.382 | 2.8 (1.5) | 2.8 (1.6) | <0.001 |
| HAS BLED Score *** | 2.0 (1.0) | 1.7 (1.1) | 0.282 | 1.8 (1.0) | 1.8 (1.0) | <0.001 |
| Hypertension | 3315 (73.7) | 1405 (65.2) | 0.185 | 885 (68.4) | 885 (68.4) | <0.001 |
| Diabetes | 1417 (31.5) | 560 (26.0) | 0.122 | 359 (27.7) | 359 (27.7) | <0.001 |
| Dyslipidemia | 1663 (36.9) | 715 (33.0) | 0.081 | 445 (34.4) | 445 (34.4) | <0.001 |
| Myocardial infarction | 121 (2.7) | 77 (3.6) | 0.069 | 40 (3.1) | 40 (3.1) | <0.001 |
| Congestive heart failure | 542 (12.1) | 249 (11.6) | 0.018 | 147 (11.4) | 147 (11.4) | <0.001 |
| Peripheral vascular disease | 253 (5.6) | 128 (5.9) | 0.013 | 72 (5.6) | 72 (5.6) | <0.001 |
| Stroke | 896 (19.9) | 335 (15.5) | 0.116 | 225 (17.4) | 225 (17.4) | <0.001 |
| CKD **** | 506 (11.2) | 191 (8.8) | 0.080 | 121 (9.4) | 121 (9.4) | <0.001 |
| Medications | ||||||
| NOAC | 3530 (78.3) | 1630 (75.3) | 0.072 | 998 (77.1) | 998 (77.1) | <0.001 |
| Warfarin | 1076 (23.9) | 642 (29.7) | 0.131 | 345 (26.7) | 345 (26.7) | <0.001 |
| Antiplatelet | 391 (8.7) | 307 (14.2) | 0.174 | 152 (11.7) | 152 (11.7) | <0.001 |
| Beta-blocker | 2446 (54.3) | 1027 (47.5) | 0.136 | 639 (49.4) | 639 (49.4) | <0.001 |
| CCB | 1360 (30.2) | 552 (25.5) | 0.104 | 351 (27.1) | 351 (27.1) | <0.001 |
| Digitalis | 459 (10.2) | 65 (3.0) | 0.292 | 52 (4.0) | 52 (4.0) | <0.001 |
| Diuretics | 362 (8.1) | 157 (7.3) | 0.030 | 97 (7.5) | 97 (7.5) | <0.001 |
| ACEi/ARB | 2015 (44.7) | 891 (41.2) | 0.071 | 543 (42.0) | 543 (42.0) | <0.001 |
| Statin | 1702 (37.8) | 804 (37.2) | 0.012 | 484 (37.5) | 484 (37.5) | <0.001 |
| Rate Control (N = 4506) | Rhythm Control (N = 2164) | |||||||
|---|---|---|---|---|---|---|---|---|
| Event, n | PYRs | Event/100 PYRs | Event, n | PYRs | Event/100 PYRs | HR (95% CI) | p-Value | |
| Primary Outcome * | 211 | 12,039 | 1.8 | 72 | 5124 | 1.4 | 0.73 (0.59~0.95) | 0.022 |
| Secondary outcome | ||||||||
| CV Death | 20 | 12,185 | 0.2 | 5 | 5262 | 0.1 | 0.53 (0.20~1.42) | 0.209 |
| Stroke | 81 | 12,087 | 0.7 | 20 | 5252 | 0.4 | 0.52 (0.32~0.85) | 0.010 |
| Acute coronary syndrome | 22 | 12,165 | 0.2 | 4 | 5267 | 0.1 | 0.40 (0.14~1.16) | 0.090 |
| HF admission | 16 | 12,180 | 0.1 | 7 | 5255 | 0.1 | 0.89 (0.37~2.18) | 0.804 |
| Rate Control (N = 1293 [4506] *) | Rhythm Control (N = 1293 [2164] *) | |||||||
|---|---|---|---|---|---|---|---|---|
| Event, n | PYRs | Event/100 PYRs | Event, n | PYRs | Event/100 PYRs | Weighted HR (95% CI) | p-Value | |
| Primary Outcome ** | 53 | 3448 | 1.5 | 45 | 3126 | 1.4 | 0.87 (0.66~1.17) | 0.363 |
| Secondary outcome | ||||||||
| CV Death | 5 | 3485 | 0.1 | 3 | 3158 | 0.1 | 0.70 (0.25~1.99) | 0.502 |
| Stroke | 20 | 3461 | 0.6 | 12 | 3150 | 0.4 | 0.60 (0.36~1.02) | 0.060 |
| Acute coronary syndrome | 6 | 3481 | 0.2 | 2 | 3159 | 0.1 | 0.45 (0.15~1.36) | 0.155 |
| HF admission | 4 | 3484 | 0.1 | 4 | 3153 | 0.1 | 0.93 (0.35~2.45) | 0.882 |
| Rate Control (N = 1293 [4506] *) | Rhythm Control (N = 1293 [2164] *) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Event, n | PYRs | Event/100 PYRs | Event, n | PYRs | Event/100 PYRs | Weighted HR (95% CI) | p-Value | P for Interaction | |
| Sex | |||||||||
| Male (N = 1658) | 32 | 2180 | 1.5 | 31 | 1987 | 1.6 | 1.02 (0.72~1.44) | 0.913 | 0.190 |
| Women (N = 928) | 20 | 1268 | 1.6 | 13 | 1139 | 1.1 | 0.65 (0.39~1.09) | 0.102 | |
| Age | |||||||||
| Age < 75 (N = 1897) | 29 | 2555 | 1.1 | 29 | 2034 | 1.4 | 1.05 (0.73~1.51) | 0.801 | 0.150 |
| Age ≥ 75 (N = 689) | 24 | 893 | 2.7 | 15 | 822 | 1.8 | 0.67 (0.42~1.08) | 0.098 | |
| Onset of AF | |||||||||
| <3 month (N = 504) | 9 | 548 | 1.6 | 10 | 546 | 1.8 | 1.09 (0.59~2.04) | 0.784 | 0.430 |
| ≥3 month (N = 2082) | 44 | 2900 | 1.5 | 35 | 2580 | 1.4 | 0.83 (0.60~1.14) | 0.252 | |
| CHA2DS2-VASc score | |||||||||
| ≤2 (N = 1206) | 15 | 1662 | 0.9 | 13 | 1481 | 0.9 | 0.87 (0.51~1.47) | 0.602 | 0.933 |
| ≥3 (N = 1380) | 38 | 1786 | 2.1 | 32 | 1645 | 2.0 | 0.89 (0.64~1.25) | 0.507 | |
| Previous stroke history | |||||||||
| Yes (N = 449) | 15 | 561 | 2.7 | 13 | 559 | 2.3 | 0.87 (0.50~1.49) | 0.602 | 0.964 |
| No (N = 2137) | 37 | 2886 | 1.3 | 31 | 2567 | 1.2 | 0.88 (0.63~1.23) | 0.442 | |
| HF history | |||||||||
| Yes (N = 294) | 10 | 353 | 2.8 | 11 | 363 | 3.0 | 0.98 (0.54~1.80) | 0.956 | 0.501 |
| No (N = 2292) | 43 | 3094 | 1.4 | 34 | 2763 | 1.2 | 0.83 (0.60~1.15) | 0.259 | |
| Left atrial diameter (LAD) | |||||||||
| LAD ≤ 4 cm (N = 640) | 11 | 740 | 1.5 | 10 | 819 | 1.2 | 0.85 (0.46~1.58) | 0.612 | 0.549 |
| LAD > 4 cm (N = 1496) | 35 | 1921 | 1.8 | 28 | 1677 | 1.7 | 0.90 (0.63~1.29) | 0.566 | |
| LAVI (mL/m2) | |||||||||
| LAVI ≤ 36 (N = 479) | 9 | 649 | 1.4 | 6 | 549 | 1.1 | 0.80 (0.39~1.67) | 0.553 | 0.876 |
| LAVI > 36 (N = 1087) | 29 | 1410 | 2.1 | 19 | 1181 | 1.6 | 0.79 (0.52~1.20) | 0.274 | |
| LVEF | |||||||||
| LVEF ≤ 40 (N = 117) | 3 | 126 | 2.4 | 2 | 148 | 1.4 | 0.65 (0.18~2.43) | 0.526 | 0.471 |
| LVEF > 40 (N = 2058) | 44 | 2589 | 1.7 | 37 | 2399 | 1.5 | 0.91 (0.66~1.24) | 0.541 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-G.; Park, J.; Park, J.-K.; Kang, K.-W.; Shim, J.; Kim, J.-B.; Kim, J.; Choi, E.-K.; Park, H.W.; Lee, Y.S.; et al. Rhythm Control and Cardiovascular or Cerebrovascular Outcomes in Patients with Atrial Fibrillation: A Study of the CODE-AF Registry. J. Clin. Med. 2023, 12, 4579. https://doi.org/10.3390/jcm12144579
Chung H-G, Park J, Park J-K, Kang K-W, Shim J, Kim J-B, Kim J, Choi E-K, Park HW, Lee YS, et al. Rhythm Control and Cardiovascular or Cerebrovascular Outcomes in Patients with Atrial Fibrillation: A Study of the CODE-AF Registry. Journal of Clinical Medicine. 2023; 12(14):4579. https://doi.org/10.3390/jcm12144579
Chicago/Turabian StyleChung, Ho-Gi, Junbeom Park, Jin-Kyu Park, Ki-Woon Kang, Jaemin Shim, Jin-Bae Kim, Jun Kim, Eue-Keun Choi, Hyung Wook Park, Young Soo Lee, and et al. 2023. "Rhythm Control and Cardiovascular or Cerebrovascular Outcomes in Patients with Atrial Fibrillation: A Study of the CODE-AF Registry" Journal of Clinical Medicine 12, no. 14: 4579. https://doi.org/10.3390/jcm12144579