Response to Prone Position in COVID-19 and Non-COVID-19 Patients with Severe ARDS Supported by vvECMO
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Study Settings
2.3. Population
2.4. Primary and Secondary Endpoints
2.5. vv-ECMO Management
2.6. Mechanical Ventilation Protocol during vv-ECMO
2.7. Prone Position Procedure
2.8. Data Collection
2.9. Assessment of Safety of Prone Position
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Outcomes
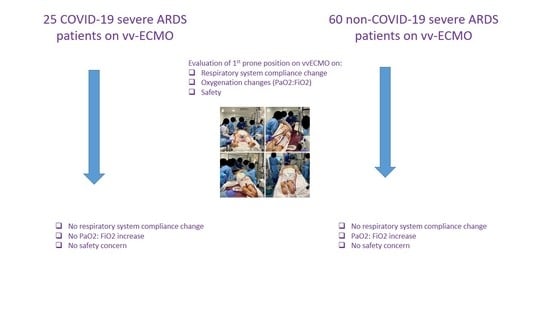
3.2. Effects of the First PP under vv-ECMO in the COVID-19 ARDS Group
3.3. Effects of the First PP under vv-ECMO in the Non-COVID-19 ARDS Group
3.4. Comparison between COVID-19 ARDS Group and Non-COVID-19 ARDS Group before and after the First PP under vv-ECMO
3.5. Assessment of Safety in the COVID-19 Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APRV | airway pressure release ventilation |
| ARDS | acute respiratory distress syndrome |
| BMI | body mass index |
| COVID-19 | coronavirus disease 2019 |
| CRS | compliance of respiratory system |
| ΔP | driving pressure |
| FiO2 | fraction of inspired oxygen |
| FmO2 | oxygen fraction delivered by the membrane oxygenator |
| ICU | intensive care unit |
| iNO | inhaled nitric oxide |
| MP | mechanical power |
| PaCO2 | partial pressure of arterial carbon dioxide |
| PaO2 | partial pressure of arterial oxygen |
| PaO2:FiO2 ratio | ratio of partial pressure of arterial oxygen to fraction of inspired oxygen |
| PEEP | positive end-expiratory pressure |
| PP | prone position |
| Ppeak | peak inspiratory pressure |
| Pplat | plateau pressure |
| RR | respiratory rate |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SP | supine position |
| SaO2 | saturation of arterial oxygen |
| VILI | ventilator-induced lung injury |
| VM | minute ventilation |
| VT | tidal volume |
| vv-ECMO | venovenous extracorporeal membrane oxygenation |
References
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.; Amato, M.B.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://covid19.who.int/ (accessed on 20 May 2023).
- Lorusso, R.; Combes, A.; Coco, V.L.; De Piero, M.E.; Belohlavek, J.; on behalf of the EuroECMO COVID-19 WorkingGroup; Euro-ELSO Steering Committee. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021, 47, 344–348. [Google Scholar] [CrossRef]
- Nesseler, N.; Fadel, G.; Mansour, A.; Para, M.; Falcoz, P.E.; Mongardon, N.; Porto, A.; Bertier, A.; Levy, B.; Cadoz, C.; et al. Extracorporeal Membrane Oxygenation for Respiratory Failure Related to COVID-19: A Nationwide Cohort Study. Anesthesiology 2022, 136, 732–748. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar] [CrossRef]
- Lorusso, R.; De Piero, M.E.; Mariani, S.; Di Mauro, M. Determinants of long-term outcomes in patients with COVID-19 supported with ECMO—Authors’ reply. Lancet Respir. Med. 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Hraiech, S.; Masson, E.; Grisoli, D.; Forel, J.M.; Boucekine, M.; Morera, P.; Guervilly, C.; Adda, M.; Dizier, S.; et al. Outcome of acute respiratory distress syndrome patients treated with extracorporeal membrane oxygenation and brought to a referral center. Intensive Care Med. 2014, 40, 74–83. [Google Scholar] [CrossRef]
- Guervilly, C.; Prud’homme, E.; Pauly, V.; Bourenne, J.; Hraiech, S.; Daviet, F.; Adda, M.; Coiffard, B.; Forel, J.M.; Roch, A.; et al. Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: Time for a randomized trial? Intensive Care Med. 2019, 45, 1040–1042. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.H.; Ramanathan, K.; Ling, R.R.; Yang, I.X.; Tan, C.S.; Schmidt, M.; Shekar, K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2021, 25, 292. [Google Scholar] [CrossRef]
- Giani, M.; Rezoagli, E.; Guervilly, C.; Rilinger, J.; Duburcq, T.; Petit, M.; Textoris, L.; Garcia, B.; Wengenmayer, T.; Bellani, G.; et al. Timing of Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Crit. Care Med. 2023, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, N.; Palumbo, D.; Cremona, G.; Vitali, G.; De Lorenzo, R.; Calvi, M.R.; Del Prete, A.; Baiardo Redaelli, M.; Calamarà, S.; Belletti, A.; et al. Residual lung damage following ARDS in COVID-19 ICU survivors. Acta Anaesthesiol. Scand. 2022, 66, 223–231. [Google Scholar] [CrossRef]
- Albert, R.K.; Keniston, A.; Baboi, L.; Ayzac, L.; Guérin, C.; Proseva Investigators. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2014, 189, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Vandenbunder, B.; Ehrmann, S.; Piagnerelli, M.; Sauneuf, B.; Serck, N.; Soumagne, T.; Textoris, J.; Vinsonneau, C.; Aissaoui, N.; Blonz, G.; et al. Static compliance of the respiratory system in COVID-19 related ARDS: An international multicenter study. Crit. Care 2021, 25, 52. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.P.; Subramaniam, A.; Chua, C.; Ling, R.R.; Anstey, C.; Ramanathan, K.; Slutsky, A.S.; Shekar, K. Respiratory system mechanics, gas exchange, and outcomes in mechanically ventilated patients with COVID-19-related acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 1178–1188. [Google Scholar] [CrossRef]
- Fusina, F.; Albani, F.; Crisci, S.; Morandi, A.; Tansini, F.; Beschi, R.; Rosano, A.; Natalini, G. Respiratory system compliance at the same PEEP level is similar in COVID and non-COVID ARDS. Respir. Res. 2022, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Camporota, L.; Marini, J.J. Prone Position and COVID-19: Mechanisms and Effects. Crit Care Med. 2022, 50, 873–875. [Google Scholar] [CrossRef]
- Rossi, S.; Palumbo, M.M.; Sverzellati, N.; Busana, M.; Malchiodi, L.; Bresciani, P.; Ceccarelli, P.; Sani, E.; Romitti, F.; Bonifazi, M.; et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022, 48, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Adda, I.; Teboul, J.L.; Persichini, R.; Gavelli, F.; Guérin, L.; Monnet, X. Effects of Prone Positioning on Venous Return in Patients with Acute Respiratory Distress Syndrome. Crit. Care Med. 2021, 49, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT04607551 (accessed on 20 May 2023).


| COVID-19 ARDS | Non-COVID-19 ARDS | p Value | |
|---|---|---|---|
| N = 25 | N = 60 | ||
| Age, median (IQR) | 55 (45–61) | 51 (38–64) | 0.79 |
| Male sex, n (%) | 18 (72) | 44 (74) | 0.80 |
| Body mass index (kg/m2), median (IQR) | 30 (27.6–35.2) | 28.7 (25.5–35.4) | 0.38 |
| SAPS 2 at admission, median (IQR) | 41 (31–49) | 47 (42–55) | 0.006 |
| SOFA score at inclusion, median (IQR) | 7 (4–9) | 10 (8–12) | 0.001 |
| Cause of ARDS | |||
| COVID-19 Viral non-COVID-19 Bacterial Aspiration Pulmonary—others Extrapulmonary sepsis | 25 (100) 0 (0) 0 (0) 0 (0) 0 (0) 0 (0) | 0 (0) 13 (22) 35 (58) 2 (3.5) 8 (13) 2 (3.5) | <0.001 |
| Comorbidity, n (%) | |||
| Immunocompromised Hypertension Diabetes mellitus Chronic renal failure Chronic obstructive pulmonary disease | 3 (12) 11 (44) 4 (16) 1 (4) 7(28) | 0 (0) 14 (24) 8 (14) 2 (3.5) 11 (19) | 0.07 0.06 0.77 0.89 0.34 |
| Before vv-ECMO | |||
| Duration of mechanical ventilation, median (IQR) Prone position, n (%) Inhaled nitric oxide, n (%) PaO2:FiO2 ratio, mmHg, median (IQR) Renal replacement therapy, n (%) | 5 (1–7) 25 (100) 20 (80) 68 (50–74) 1 (4) | 3 (1–7) 44 (74) 26 (44) 66 (50–81) 2 (3.5) | 0.47 0.005 0.002 0.93 0.89 |
| Referred from other ICUs, n (%) Retrieved by vv-ECMO mobile team, n (%) | 24 (96) 21 (84) | 56 (95) 48 (81) | 0.83 0.77 |
| vv-ECMO configuration, n (%) | |||
| Femoro-jugular Femoro-femoral Jugulo-jugular | 25 (100) 0 (0) 0 (0) | 55 (92) 4 (7) 1 (1) | 0.32 |
| Outcomes | |||
| ECMO days before PP, median (IQR) Number of PP sessions on vv-ECMO, median (IQR) vv-ECMO duration, days, median (IQR) vv-ECMO weaning rate, n (%) ICU mortality rate, n (%) Hospital mortality rate, n (%) | 2 (1–3) 4 (3–6) 23 (15–34) 18 (72) 12 (48) 12 (48) | 5 (3–7) 2 (1–4) 20 (13–36) 38 (64) 32 (54) 36 (61) | <0.001 <0.001 0.75 0.50 0.60 0.27 |
| Baseline Supine H-1 PP | Start of Prone H+1 PP | End of Prone H-1 SP | Return to Supine H+1 SP | p Value | |
|---|---|---|---|---|---|
| Ventilatory parameters | |||||
| Tidal volume, mL, median (IQR) | 150 (106–215) | 145 (100–220) | 150 (115–200) | 160 (100–230) | 0.97 |
| Plateau airway pressure, cm H2O, median (IQR) | 25 (21–26) | 25 (22–26) | 23 (23–24) | 25 (22–26) | 0.48 |
| Peak inspiratory pressure, cm H2O, median (IQR) | 27 (23–29) | 29 (26–32) | 26 (25–30) | 29 (25–31) | 0.36 |
| PEEP, cm H2O, median (IQR) | 12 (9–14) | 12 (10–14) | 12 (10–14) | 12 (10–14) | 0.93 |
| Driving pressure, cm H2O, median (IQR) | 12 (10–15) | 13 (9–15) | 12 (8–14) | 13 (11–14) | 0.68 |
| Respiratory rate, cycles/min, median (IQR) | 15 (13–17) | 15 (13–16) | 15 (12–16) | 15 (13–19) | 0.89 |
| Minute ventilation, L/min, median (IQR) | 2.2 (1.5–3.7) | 2 (1.5–3.6) | 2.1 (1.5–3.4) | 2.4 (1.5–3.8) | 0.94 |
| Respiratory system compliance, mL/cm H2O, median (IQR) | 11 (10–17) | 13 (10–21) | 13 (10–21) | 11 (9–17) | 0.83 |
| Mechanical power, J/min, median (IQR) | 4.1 (2.8–7.2) | 4.7 (3.4–9) | 4.2 (3.1–8.2) | 4.3 (3.4–9) | 0.94 |
| Inspired fraction of oxygen, %, median (IQR) | 50 (40–75) | 60 (45–80) | 50 (40–70) | 55 (35–75) | 0.76 |
| vv-ECMO parameters | |||||
| vv-ECMO blood flow, L/min, median (IQR) | 3.8 (3.3–4.7) | 4 (3.3–4.4) | 3.8 (3.2–4.8) | 3.9 (3.2–4.6) | 0.99 |
| Sweep gas flow, L/min, median (IQR) | 5 (3.5–6) | 5 (4–6) | 5 (3.5–7) | 5 (3.7–6.5) | 0.93 |
| Membrane lung fraction of oxygen, %, median (IQR) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 100 (100–100) | - |
| Arterial blood gas | |||||
| PaO2, mmHg, median (IQR) | 75 (69–81) | 78 (69–85) | 77 (70–83) | 77 (67–89) | 0.33 |
| PaCO2, mmHg, median (IQR) | 54 (43–58) | 51 (43–55) | 53 (45–56) | 48 (44–54) | 0.57 |
| PaO2:FiO2 ratio, mmHg, median (IQR) | 140 (95–185) | 142 (98–186) | 144 (127–207) | 147 (95–221) | 0.74 |
| pH, median (IQR) | 7.40 (7.36–7.42) | 7.42 (7.37–7.43) | 7.40 (7.35–7.44) | 7.42 (7.39–7.45) | 0.11 |
| Hemodynamic parameters | |||||
| Heart rate, bpm, median (IQR) | 89 (71–116) | 92 (73–111) | 96 (76–105) | 81 (70–105) | 0.70 |
| Mean arterial pressure, mmHg, median (IQR) | 80 (73–90) | 87 (80–100) * | 87 (77–100) * | 73 (67–86) | 0.002 |
| Supine before Proning | Supine after Proning | p Value | |
|---|---|---|---|
| Ventilatory parameters | |||
| Tidal volume, mL, mean ± sd | 206 ± 110 | 201 ± 99 | 0.79 |
| Plateau airway pressure, cm H2O, mean ± sd | 25 ± 4 | 25 ± 4 | 0.21 |
| PEEP, cm H2O, mean ± sd | 15 ± 3 | 15 ± 3 | 0.85 |
| Driving pressure, cm H2O, mean ± sd | 11 ± 4 | 10 ± 4 | 0.28 |
| Respiratory rate, cycles/min, mean ± sd | 14 ± 6 | 13 ± 5 | 0.79 |
| Minute ventilation, L/min, mean ± sd | 2.9 ± 2.1 | 2.8 ± 2.2 | 0.84 |
| Respiratory system compliance, mL/cm H2O, mean ± sd | 22.4 ± 12.3 | 22.5 ± 12.3 | 0.95 |
| Inspired fraction of oxygen, %, mean ± sd | 63 ± 22 | 54 ± 18 | 0.022 |
| vv-ECMO parameters | |||
| vv-ECMO blood flow, L/min, mean ± sd | 4 ± 0.8 | 3.8 ± 0.8 | 0.35 |
| Sweep gas flow, L/min, mean ± sd | 6 ± 2 | 6 ± 2 | 0.90 |
| Membrane lung fraction of oxygen, %, mean ± sd | 100 ± 0 | 100 ± 0 | - |
| Arterial blood gas | |||
| PaO2, mmHg, mean ± sd | 75 ± 14 | 84 ± 22 | 0.002 |
| PaCO2, mmHg, mean ± sd | 45 ± 10 | 43 ± 9 | 0.32 |
| PaO2:FiO2 ratio, mmHg, mean ± sd | 135 ± 57 | 176 ± 72 | 0.001 |
| Supine before Proning | p Value | Supine after Proning | p Value | |||
|---|---|---|---|---|---|---|
| COVID-19 ARDS N = 25 | Non-COVID-19 ARDS N = 60 | COVID-19 ARDS N = 25 | Non-COVID-19 ARDS N = 60 | |||
| Ventilatory parameters | ||||||
| Tidal volume, mL, median (IQR) | 150 (106–215) | 170 (150–243) | 0.08 | 160 (115–240) | 170 (130–250) | 0.31 |
| Plateau airway pressure, cm H2O, median (IQR) | 25 (21–26) | 26 (23–28) | 0.06 | 25 (22–26) | 25 (22–26) | 0.90 |
| PEEP, cm H2O, median (IQR) | 12 (9–14) | 15 (12–18) | <0.001 | 12 (10–14) | 15 (12–18) | 0.002 |
| Driving pressure, cm H2O, median (IQR) | 12 (10–15) | 10 (7–13) | 0.06 | 13 (11–14) | 9 (7–12) | 0.001 |
| Respiratory rate, cycles/min, median (IQR) | 15 (13–17) | 12 (10–15) | 0.01 | 15 (13–19) | 12 (10–15) | 0.006 |
| Minute ventilation, L/min, median (IQR) | 2.2 (1.5–3.7) | 2.1 (1.5–3.3) | 0.84 | 2.4 (1.5–3.8) | 2.0 (1.5–3.0) | 0.44 |
| Respiratory system compliance, mL cm H2O, median (IQR) | 11 (10–17) | 20 (12–31) | 0.009 | 11 (9–17) | 21 (13–30) | 0.005 |
| Inspired fraction of oxygen, %, median (IQR) | 50 (40–75) | 60 (40–80) | 0.19 | 55 (35–75) | 50 (40–60) | 0.35 |
| ECMO parameters | ||||||
| vv-ECMO blood flow, L/min, median (IQR) | 3.8 (3.3–4.7) | 3.8 (3.2–4.6) | 0.76 | 3.9 (3.2–4.5) | 3.7 (3.2–4.5) | 0.53 |
| Sweep gas flow, L/min, median (IQR) | 5 (3.5–6) | 6 (5–7) | 0.04 | 5 (4–6) | 6 (5–7) | 0.24 |
| Membrane lung fraction of oxygen, %, median (IQR) | 100 (100–100) | 100 (100–100) | 1 | 100 (100–100) | 100 (100–100) | 1 |
| Arterial blood gas | ||||||
| PaO2, mmHg, median (IQR) | 75 (69–81) | 71 (64–82) | 0.42 | 77 (67–89) | 77 (68–92) | 0.66 |
| PaCO2, mmHg, median (IQR) | 54 (43–58) | 43 (39–49) | 0.006 | 48 (44–54) | 42 (38–50) | 0.008 |
| PaO2:FiO2 ratio, mmHg, median (IQR) | 140 (95–185) | 127 (92–162) | 0.27 | 147 (95–221) | 160 (125–214) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Textoris, L.; Gragueb-Chatti, I.; Daviet, F.; Valera, S.; Sanz, C.; Papazian, L.; Forel, J.-M.; Hraiech, S.; Roch, A.; Guervilly, C. Response to Prone Position in COVID-19 and Non-COVID-19 Patients with Severe ARDS Supported by vvECMO. J. Clin. Med. 2023, 12, 3918. https://doi.org/10.3390/jcm12123918
Textoris L, Gragueb-Chatti I, Daviet F, Valera S, Sanz C, Papazian L, Forel J-M, Hraiech S, Roch A, Guervilly C. Response to Prone Position in COVID-19 and Non-COVID-19 Patients with Severe ARDS Supported by vvECMO. Journal of Clinical Medicine. 2023; 12(12):3918. https://doi.org/10.3390/jcm12123918
Chicago/Turabian StyleTextoris, Laura, Ines Gragueb-Chatti, Florence Daviet, Sabine Valera, Céline Sanz, Laurent Papazian, Jean-Marie Forel, Sami Hraiech, Antoine Roch, and Christophe Guervilly. 2023. "Response to Prone Position in COVID-19 and Non-COVID-19 Patients with Severe ARDS Supported by vvECMO" Journal of Clinical Medicine 12, no. 12: 3918. https://doi.org/10.3390/jcm12123918