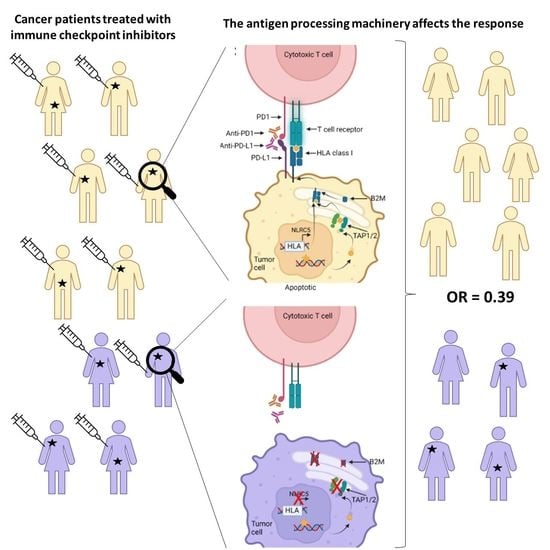
Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Literature Search
2.2. Data Extraction
2.3. Definitions
2.4. Clinical Outcome
2.5. Redundant Data Sets
2.6. Statistical Analyses
3. Results
3.1. The Literature Search
3.2. Cohort Presentation
Meta-Analyses
3.3. Case Presentations
3.3.1. Response to ICI Therapy
3.3.2. ICI Resistance Acquired via APM Regulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients With Advanced Non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The Foundations of Immune Checkpoint Blockade and the Ipilimumab Approval Decennial. Nat. Rev. Drug Discov. 2022, 21, 509–528. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]
- Larouche, V.; Atkinson, J.; Albrecht, S.; Laframboise, R.; Jabado, N.; Tabori, U.; Bouffet, E. International bMMRD consortium Sustained Complete Response of Recurrent Glioblastoma to Combined Checkpoint Inhibition in a Young Patient with Constitutional Mismatch Repair Deficiency. Pediatr. Blood Cancer 2018, 65, e27389. [Google Scholar] [CrossRef]
- Yang, Y.; Jain, R.K.; Glenn, S.T.; Xu, B.; Singh, P.K.; Wei, L.; Hu, Q.; Long, M.; Hutson, N.; Wang, J.; et al. Complete Response to Anti-PD-L1 Antibody in a Metastatic Bladder Cancer Associated with Novel MSH4 Mutation and Microsatellite Instability. J. Immunother. Cancer 2020, 8, e000128. [Google Scholar] [CrossRef]
- Zhang, N.; Zhu, J.; Lv, H. Complete Response to Pembrolizumab in a Patient with Extensive-Stage Small-Cell Lung Cancer: A Case Report. Ann. Palliat. Med. 2020, 9, 2342352–2347352. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Sosman, J.A.; Atkins, M.B.; Leming, P.D.; et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019, 5, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Wang, S.; He, Z.; Wang, X.; Li, H.; Liu, X.-S. Antigen Presentation and Tumor Immunogenicity in Cancer Immunotherapy Response Prediction. eLife 2019, 8, e49020. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of Resistance to Immune Checkpoint Inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, M.; Janikovits, J.; Von Knebel Doeberitz, M.; Kloor, M. Complex Pattern of Immune Evasion in MSI Colorectal Cancer. Oncoimmunology 2018, 7, e1445453. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA Mutanome Vaccines Mobilize Poly-Specific Therapeutic Immunity against Cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ladányi, A.; Papp, E.; Mohos, A.; Balatoni, T.; Liszkay, G.; Oláh, J.; Varga, A.; Lengyel, Z.; Emri, G.; Ferrone, S. Role of the Anatomic Site in the Association of HLA Class I Antigen Expression Level in Metastases with Clinical Response to Ipilimumab Therapy in Patients with Melanoma. J. Immunother. Cancer 2020, 8, e000209. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC Proteins Confer Differential Sensitivity to CTLA-4 and PD-1 Blockade in Untreated Metastatic Melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef] [Green Version]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to Checkpoint Blockade Therapy through Inactivation of Antigen Presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H.; Kim, H.S.; Cha, H.; Kim, S.; Kim, T.M.; Anagnostou, V.; Choi, Y.-L.; Jung, H.A.; Sun, J.-M.; Ahn, J.S.; et al. HLA-Corrected Tumor Mutation Burden and Homologous Recombination Deficiency for the Prediction of Response to PD-(L)1 Blockade in Advanced Non-Small-Cell Lung Cancer Patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 902–911. [Google Scholar] [CrossRef]
- Thompson, J.C.; Davis, C.; Deshpande, C.; Hwang, W.-T.; Jeffries, S.; Huang, A.; Mitchell, T.C.; Langer, C.J.; Albelda, S.M. Gene Signature of Antigen Processing and Presentation Machinery Predicts Response to Checkpoint Blockade in Non-Small Cell Lung Cancer (NSCLC) and Melanoma. J. Immunother. Cancer 2020, 8, e000974. [Google Scholar] [CrossRef] [PubMed]
- Yoshihama, S.; Cho, S.X.; Yeung, J.; Pan, X.; Lizee, G.; Konganti, K.; Johnson, V.E.; Kobayashi, K.S. NLRC5/CITA Expression Correlates with Efficient Response to Checkpoint Blockade Immunotherapy. Sci. Rep. 2021, 11, 3258. [Google Scholar] [CrossRef]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A Single Dose of Neoadjuvant PD-1 Blockade Predicts Clinical Outcomes in Resectable Melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Gide, T.N.; Quek, C.; Menzies, A.M.; Tasker, A.T.; Shang, P.; Holst, J.; Madore, J.; Lim, S.Y.; Velickovic, R.; Wongchenko, M.; et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell 2019, 35, 238–255. [Google Scholar] [CrossRef]
- Cindy Yang, S.Y.; Lien, S.C.; Wang, B.X.; Clouthier, D.L.; Hanna, Y.; Cirlan, I.; Zhu, K.; Bruce, J.P.; El Ghamrasni, S.; Iafolla, M.A.J.; et al. Pan-Cancer Analysis of Longitudinal Metastatic Tumors Reveals Genomic Alterations and Immune Landscape Dynamics Associated with Pembrolizumab Sensitivity. Nat. Commun. 2021, 12, 5137. [Google Scholar] [CrossRef]
- Giroux Leprieur, E.; Hélias-Rodzewicz, Z.; Takam Kamga, P.; Costantini, A.; Julie, C.; Corjon, A.; Dumenil, C.; Dumoulin, J.; Giraud, V.; Labrune, S.; et al. Sequential CtDNA Whole-Exome Sequencing in Advanced Lung Adenocarcinoma with Initial Durable Tumor Response on Immune Checkpoint Inhibitor and Late Progression. J. Immunother. Cancer 2020, 8, e000527. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative Immune Checkpoint Regulation by VISTA: A Mechanism of Acquired Resistance to Anti-PD-1 Therapy in Metastatic Melanoma Patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Seremet, T.; Koch, A.; Jansen, Y.; Schreuer, M.; Wilgenhof, S.; Del Marmol, V.; Liènard, D.; Thielemans, K.; Schats, K.; Kockx, M.; et al. Molecular and Epigenetic Features of Melanomas and Tumor Immune Microenvironment Linked to Durable Remission to Ipilimumab-Based Immunotherapy in Metastatic Patients. J. Transl. Med. 2016, 14, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic Correlates of Response to CTLA-4 Blockade in Metastatic Melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-Specific MHC-II Expression Represents a Tumour-Autonomous Phenotype and Predicts Response to Anti-PD-1/PD-L1 Therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, B.B.Y.; Lim, W.-T.; Goh, B.-C.; Hui, E.P.; Lo, K.-W.; Pettinger, A.; Foster, N.R.; Riess, J.W.; Agulnik, M.; Chang, A.Y.C.; et al. Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1412–1418. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Shi, W.; Zhu, M.; Liu, Z.; Luo, N.; Zeng, Y.; He, Y. Serial Ultra-Deep Sequencing of Circulating Tumor DNA Reveals the Clonal Evolution in Non-Small Cell Lung Cancer Patients Treated with Anti-PD1 Immunotherapy. Cancer Med. 2019, 8, 7669–7678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurkmans, D.P.; Kuipers, M.E.; Smit, J.; van Marion, R.; Mathijssen, R.H.J.; Postmus, P.E.; Hiemstra, P.S.; Aerts, J.G.J.V.; von der Thüsen, J.H.; van der Burg, S.H. Tumor Mutational Load, CD8+ T Cells, Expression of PD-L1 and HLA Class I to Guide Immunotherapy Decisions in NSCLC Patients. Cancer Immunol. Immunother. CII 2020, 69, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yang, M.; Li, K.; Li, J.; Xu, L.; Xu, F.; Xu, Y.; Ren, D.; Zhang, J.; Liu, L. Immune-Related Genes and Gene Sets for Predicting the Response to Anti-Programmed Death 1 Therapy in Patients with Primary or Metastatic Non-Small Cell Lung Cancer. Oncol. Lett. 2021, 22, 540. [Google Scholar] [CrossRef]
- Busch, E.; Ahadova, A.; Kosmalla, K.; Bohaumilitzky, L.; Pfuderer, P.L.; Ballhausen, A.; Witt, J.; Wittemann, J.-N.; Bläker, H.; Holinski-Feder, E.; et al. Beta-2-Microglobulin Mutations Are Linked to a Distinct Metastatic Pattern and a Favorable Outcome in Microsatellite-Unstable Stage IV Gastrointestinal Cancers. Front. Oncol. 2021, 11, 669774. [Google Scholar] [CrossRef]
- Sinn, B.V.; Loibl, S.; Hanusch, C.A.; Zahm, D.-M.; Sinn, H.-P.; Untch, M.; Weber, K.; Karn, T.; Becker, C.; Marmé, F.; et al. Immune-Related Gene Expression Predicts Response to Neoadjuvant Chemotherapy but Not Additional Benefit from PD-L1 Inhibition in Women with Early Triple-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 2584–2591. [Google Scholar] [CrossRef]
- Sabbatino, F.; Marra, A.; Liguori, L.; Scognamiglio, G.; Fusciello, C.; Botti, G.; Ferrone, S.; Pepe, S. Resistance to Anti-PD-1-Based Immunotherapy in Basal Cell Carcinoma: A Case Report and Review of the Literature. J. Immunother. Cancer 2018, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Khaddour, K.; Dowling, J.; Huang, J.; Council, M.; Chen, D.; Cornelius, L.; Johanns, T.; Dahiya, S.; Ansstas, G. Successful Administration of Sequential TVEC and Pembrolizumab Followed by Temozolomide in Immunotherapy Refractory Intracranial Metastatic Melanoma with Acquired B2M Mutation. Oncotarget 2020, 11, 4836–4844. [Google Scholar] [CrossRef]
- Czink, E.; Kloor, M.; Goeppert, B.; Frohling, S.; Uhrig, S.; Weber, T.F.; Meinel, J.; Sutter, C.; Weiss, K.H.; Schirmacher, P.; et al. Successful Immune Checkpoint Blockade in a Patient with Advanced Stage Microsatellite-Unstable Biliary Tract Cancer. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001974. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, W.; Zhao, H.; Zeng, Z.; Zhang, F.; Hu, Y.; Li, Q.; Chen, J.; Meng, E.; Xiao, W. Homozygous Deletion of the HLA-B Gene as an Acquired-Resistance Mechanism to Nivolumab in a Patient with Lung Adenocarcinoma: A Case Report. Ann. Transl. Med. 2021, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Richmond, C.S.; Vallatharasu, Y.; Deviley, J.A.; Vos, C.R.; Parsons, B.M.; Kenny, P.A. Sequential Treatment Failures in Response to BRAF/MEK and Immune Checkpoint Inhibitors Mediated by MAP2K2 and B2M Mutations in Melanoma. Exp. Mol. Pathol. 2019, 110, 104260. [Google Scholar] [CrossRef]
- Gurjao, C.; Liu, D.; Hofree, M.; AlDubayan, S.H.; Wakiro, I.; Su, M.J.; Felt, K.; Gjini, E.; Brais, L.K.; Rotem, A.; et al. Intrinsic Resistance to Immune Checkpoint Blockade in a Mismatch Repair-Deficient Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Ugurel, S.; Spassova, I.; Wohlfarth, J.; Drusio, C.; Cherouny, A.; Melior, A.; Sucker, A.; Zimmer, L.; Ritter, C.; Schadendorf, D.; et al. MHC Class-I Downregulation in PD-1/PD-L1 Inhibitor Refractory Merkel Cell Carcinoma and Its Potential Reversal by Histone Deacetylase Inhibition: A Case Series. Cancer Immunol. Immunother. CII 2019, 68, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yu, M.; Zhou, L.; Jiang, L.; Huang, M. Durable Response to Combination Radiotherapy and Immunotherapy in EP-Resistant Lung Large-Cell Neuroendocrine Carcinoma with B2M and STK11 Mutations: A Case Report. Immunotherapy 2020, 12, 223–227. [Google Scholar] [CrossRef]
- Balatoni, T.; Mohos, A.; Papp, E.; Sebestyén, T.; Liszkay, G.; Oláh, J.; Varga, A.; Lengyel, Z.; Emri, G.; Gaudi, I.; et al. Tumor-Infiltrating Immune Cells as Potential Biomarkers Predicting Response to Treatment and Survival in Patients with Metastatic Melanoma Receiving Ipilimumab Therapy. Cancer Immunol. Immunother. CII 2018, 67, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Sabbatino, F.; Liguori, L.; Polcaro, G.; Salvato, I.; Caramori, G.; Salzano, F.A.; Casolaro, V.; Stellato, C.; Col, J.D.; Pepe, S. Role of Human Leukocyte Antigen System as A Predictive Biomarker for Checkpoint-Based Immunotherapy in Cancer Patients. Int. J. Mol. Sci. 2020, 21, 7295. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus Chemotherapy versus Chemotherapy Alone in Non-Small Cell Lung Cancer: A Randomized, Controlled, Double-Blind Phase 3 Trial. Nat. Med. 2022, 28, 2374–2380. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, H.; Xiao, Y.; Wang, R.; Zhang, L.; Huang, C.; Cao, Y.; Sun, C.; Zhao, Y.; Lin, H.; et al. Durable Response to Immunotherapy With Antiangiogenic Drug in Large-Cell Lung Carcinoma With Multiple Fulminant Postoperative Metastases: A Case Report. Front. Oncol. 2021, 11, 633446. [Google Scholar] [CrossRef]
- Ritter, C.; Fan, K.; Paschen, A.; Reker Hardrup, S.; Ferrone, S.; Nghiem, P.; Ugurel, S.; Schrama, D.; Becker, J.C. Epigenetic Priming Restores the HLA Class-I Antigen Processing Machinery Expression in Merkel Cell Carcinoma. Sci. Rep. 2017, 7, 2290. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]





| Cohort [Reference with Relevant Data] | Patients (n) Treated/ Analyzed | Tumor Type | ICI Treatment | Response Definition | ORR | CB | PFS (Median Months) | OS (Median Months) | Biomarker | Summary | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Van Allen et al., 2015 [36] a | 110/105 | Metastatic melanoma | Ipi | CR, PR, and SD | NA | APM−: 11% APM+: 29% | NA | NA | Mutations and RNA expression of HLA-A, HLA-B, and HLA-C | B2M LOH was not significantly associated with survival | CR, PR, and SD or no clinical benefit with long-term survival |
| Hugo et al., 2016 [28] a | 38/38 | Metastatic melanoma | Pembro or nivo | CR, PR, and SD | NA | APM−: 29% APM+: 61% | NA | NA | Mutations in B2M | B2M LOH was significantly associated with worse OS | |
| Zaretsky et al., 2016 [34] b | 78/4 | Metastatic melanoma | Pembro | CR and PR | APM−: 100% APM+: 100% | APM−: 100% APM+: 100% | APM−: 19.5 APM+: 21.4 | APM−: 31.5 APM+: 32.0 | Mutations in B2M and NLRC5 and protein expression of HLA-A, HLA-B, and HLA-C | Patient 3 had a frame-shift deletion in B2M and loss of HLA class I protein expression. In supplementary data it could be seen that patient 2 had a missense mutation in NLRC5 in the relapse tumor. 2/4 with PR followed by PD, had a mutation in the APM | Responses and PFS recorded prior to PD |
| Seremet et al., 2016 [35] | 39/4 | Metastatic melanoma | Ipi or ipi + a dendritic cell vaccine | CR, PR, and SD c | APM−: 0% | APM−: 0% | NA | APM−: 13 | Protein expression of HLA class I and TAP1 | Four patients (MEL15, MEL21, MEL22, and MEL26) with no clinical benefit showed HLA class I and/or TAP1 expression | HLA class I and TAP1 expression were unknown for the remaining samples |
| Johnson et al., 2016 [37] | 30/28 | Metastatic melanoma | Nivo or pembro or atezo | CR, PR, and SD d | NA | NA | NA | NA | Protein expression of HLA-A | HLA-A expression level was not statistically associated with response to therapy | HLA-A positivity was not defined hampering detailed data extraction |
| Kakavand et al., 2017 [33] b | 44911 | Metastatic melanoma | Pembro, nivo, or ipi + pembro | CR, PR, and SD d | APM−: 100% APM+: 75% | APM−: 100% APM+: 100% | AMP−: 5.9 APM+: 14.9 | NA | Protein expression of HLA-A | 4/12 patients (33%) that progressed had a decrease of HLA-A protein expression | Responses and PFS recorded prior to PD |
| Ma et al., 2018 [38] | 44/41 | Recurrent or metastatic nasopharyngeal carcinoma | Nivo | CR, PR, and SD c | APM−: 33% APM+: 15% | NA | APM−: 4.8 APM+: 1.8 | APM−: NR APM+: 10.9 | Protein expression of HLA-A and HLA-B | HLA-A and HLA-B expression did not predict response to nivo but there was a statistical association between loss of expression of HLA-A and/or HLA-B and PFS | Median 1-year PFS and OS |
| Sade-Feldman et al., 2017 [24] | 17/17 | Metastatic melanoma | Anti-CTLA-4, anti-PD1, or anti-PD-L1 | Regression | NA | APM−: 10% APM+: 57% | NA | NA | Mutations in TAP1, TAP2, B2M, HLA-A, HLA-B, HLA-C, and protein expression of B2M and HLA-A, HLA-B, and HLA-C | Mutations in TAP1/2 were found in both non-responders and responders. 5 out of 17 patients (29%) exhibited B2M defects of which 3/5 initially responded and then progressed (Pat208 with LOH and frameshift mutation (and loss of protein expression, also of HLA class I); Pat33 with frameshift mutations; Pat99 with LOH and loss of HLA protein expression). 2/7 non-responders had B2M LOH (Pat25 (also with loss of HLA protein expression) and Pat115). Loss of both copies was only observed for non-responders. No B2M alterations were detected in responders within their cohort. There was no difference in HLA expression between responders and non-responders | CB was based on either somatic mutation or LOH or both in regard to progression/regression for all the relevant biomarkers and was scored by the authors |
| Li et al., 2019 [39] | 60/5 | Advanced NSCLC | Pembro | CR, PR, and SD | APM−: 0% APM+: 25% | APM−: 0% APM+: 25% | APM−: 7.0 APM+: 5.0 | NA | Mutations in B2M | During the partial response for patient 8, two B2M mutations were identified. She had progressive disease after 30 weeks | |
| Rodig et al., 2018 [23] | 280/181 | Advanced malignant melanoma | Ipi, nivo + ipi | CR, PR, and SD c | APM−: 33% APM+: 27% | APM−: 39% APM+: 27% | NA | NA | Protein and RNA expression of B2M, HLA-A, HLA-B, HLA-C, and TAP2 | Reduced HLA class I expression was associated with primary resistance to ipi, but not to nivo. A gene set score derived from the top 25 differentially expressed genes (including TAP2) was significantly higher in tumor samples from patients without PD compared with those with PD at week 13 after single-agent nivolumab (CheckMate 064). For patients initially treated with ipi, low baseline tumor HLA class I expression (≤50%) was associated with inferior OS. No amount of tumor HLA class I expression identified a population with inferior OS when initially treated with nivo | Data from both CheckMate 064 and 069 were included. ORR and CB could, however, only be calculated for 40 patients from the CheckMate 064 study using data from TAP2 RNA expression scored by the reviewing authors |
| Huang et al., 2019 [29] e | 14/14 | Advanced malignant melanoma | Pembro | No recurrence | NA | APM−: 29% APM+: 100% | NA | NA | RNA expression of B2M and TAP1 among others | Patients with no recurrence had a significantly higher APM score than those with recurrence with a median follow-up of 25 months. Disease-free survival was significantly longer in patients with upregulation in APM genes | APM+ was defined as an AMP Z score above 0 |
| Giroux Leprieur et al., 2020 [32] f | 79/8 | Advanced NSCLC | Nivo or pembro | CR, PR, and SD c | APM−: 100% APM+: 100% | APM−: 100%, APM+: 100% | NA | NA | LOH of B2M, HLA-A, HLA-B | LOH of HLA-A and HLA-B in patient #1. LOH of B2M in patient #3 | Responses recorded prior to PD |
| Ladányi et al., 2020 [22] | 30/30 | Metastatic melanoma | Ipi | CR, PR, and SD c,d | APM−: 0%, APM+: 43% | APM−: 25% APM+: 50% | NA | NA | Protein expression of HLA-A, HLA-B, B2M | HLA class I antigen expression level in lymph node metastases, but not in cutaneous or subcutaneous metastases, was significantly correlated to clinical response and to patients’ OS. When evaluated in all metastases analyzed, it was not significantly associated with OS | APM− is defined as low expression of ≥2 APM biomarkers |
| Shim et al., 2020 [25] | 198/198 | Advanced NSCLC | Nivo, pembro, or anti-PD-L1 | CR, PR, and SD d | APM−: 32%, APM+: 31% | APM−: 57%, APM+: 57% | NA | NA | LOH of HLA class I | No association between HLA-LOH and response to the anti-PD1 /anti-PD-L1 agent. Reanalysis of the Van Allen cohort (110 melanoma patients) found the same | |
| Hurkmans et al., 2020 [40] | 99/29 | Advanced NSCLC | Nivo | CR, PR, and SD c,g | APM−: 0% APM+: 11% | APM−: 27% APM+: 39% | NA | NA | Protein expression of HLA-A and HLA-B/C | HLA class I as an individual biomarker was not significantly associated with better OS or PFS. Patients with complete loss had impaired PFS compared to patients with partial loss or normal expression of HLA class I. No significant association was found for HLA class I and response groups | |
| Thompson et al., 2020 [26] | 67/51 | Metastatic NSCLC | Nivo, pembro, or atezo | CR, PR, and SD d | NA | APM−: 26% APM+: 63% | APM−: 1.74 APM+: 18.1 | APM−: 6.3 APM+: 19.7 | RNA expression of B2M, TAP, and NLRC5 | Higher expression of APM in the responder group compared with non-responders. APM score above the median value for the entire cohort was associated with significantly improved PFS and OS | For CB, downregulated expression was scored as APM− by the reviewing authors |
| Cindy Yang et al., 2021 [31] | 106/48 | 30 advanced solid cancer types h | Pembro | CR, PR, and SD c,i | NA | APM−: 50% APM+: 62% | NA | NA | Mutations in B2M, TAP1, TAP2, and HLA-A | B2M LOH corresponded with resistance. No notable associations between the frequency of somatic LOH events in HLA class I genes and pembro therapeutic benefit | For CB, cases were scored by the reviewing authors as AMP- when at least one relevant gene was mutated |
| Chen et al., 2021 [41] | 24/11 | Metastatic NSCLC | Nivo or durva j | CR, PR, and SD | NA | APM−: 17% APM+: 80% | NA | NA | RNA expression of HLA-A | Upregulation of HLA-A is associated with longer PFS and may be applied to predict the efficacy in patients with metastatic non-small cell lung cancer | |
| Busch et al., 2021 [42] | 19/18 | MSI metastatic GI cancers k | Pembro or nivo + ipi | CR, PR, and SD c | APM−: 25% APM+: 50% | APM−: 100% APM+: 79% | APM−: 19.5 APM+: 33.0 | NA | Mutations in B2M | No significant differences in therapy best response and survival were observed between B2M-mutant tumors, all of which also had immunohistochemical loss of B2M, and B2M-wild type tumor patients | |
| Sinn et al., 2021 [43] | 88/83 | Early triple-negative breast cancer | Durva + chemotherapy | pCR | NA | NA | NA | NA | RNA expression of HLA-A, HLA-B, TAP1 | High expression of eight genes (including TAP1, HLA-A, and HLA-B) were significantly associated with response | |
| Gide et al., 2019 [30] l | 63/41 | Malignant melanoma | Nivo or pembro | CR, PR, and SD m,n | NA | APM−: 42% APM+: 64% | NA | NA | RNA expression of HLA-A, HLA-B, HLA-C, B2M, TAP1, and NLRC5 | The responder group exhibited higher expression of NLRC5 and HLA-B than the non-responder group, and B2M showed a similar trend although it was not statistically significant | For CB, downregulated expression was scored as APM− by the reviewing authors |
| Case [Reference] | Tumor Investigated | ICI Treatment | Objective Response | PFS (Months) | OS (Months) | Biomarker Result | Comment |
|---|---|---|---|---|---|---|---|
| Czink et al., 2017 [46] | Recurrent extrahepatic cholangiocarcinoma | Pembro | SD | 13 | 13 | Normal expression of HLA class I and B2M | |
| Zhang et al., 2021 [47] | Lung adenocarcinoma | Nivo | PD | 15 | 15 | Homozygote HLA-B deletion | ICI treatment was ended due to adverse events |
| Richmond et al., 2019 [48] | Recurrent malignant melanoma | Nivo and pembro | PD | 24 | 24 | Loss of function B2M mutation | |
| Gurjao et al., 2019 [49] | Metastatic colorectal cancer | Pembro | PD | 2 | 5 | B2M frameshift mutation and LOH | |
| Ugurel et al., 2019 #1 [50] | Merkel cell carcinoma metastases | Avelu | PD | 1 a | 1 a | Loss of HLA class I protein expression | |
| Ugurel et al., 2019 #2 [50] | Merkel cell carcinoma | Nivo | PD | 6 | 6 | Low protein expression of HLA class I | |
| Ugurel et al., 2019 #3 [50] | Merkel cell carcinoma metastases | Nivo | PD | 14 | 35 | Low protein expression of HLA class I | |
| Ugurel et al., 2019 #4 [50] | Recurrent Merkel cell carcinoma metastasis | Pembro | PD | 9 | 16 | No HLA class I expression | |
| Sahin et al., 2017 [21] | Recurrent malignant melanoma metastases | Nivo | PD | 3 | 3.2 b | Homozygote B2M deletion | Part of a phase I study |
| Sabbatino et al., 2018 [44] | Basal cell carcinoma | Nivo | CR | 19 | 19 | Lack of HLA class I and B2M protein expression | Patient originally presented with a non-small cell lung cancer |
| Khaddour et al., 2020 [45] | Recurrent metastatic malignant melanoma | Combined nivo and ipi | CR | 30 | 30 | No B2M mutation | Patient had CR for 30 months before lung metastases developed at 30 months |
| Khaddour et al., 2020 [45] | Malignant melanoma-induced lung metastasis | Combined nivo and ipi followed by pembro (combined with TVEC and TMZ) | Good response | 3 | 5 | Loss of function B2M mutation | Pembrolizumab treatment was ended after 2 months due to verified B2M mutation |
| Qin et al., 2020 [51] | Metastatic large cell neuroendocrine carcinoma in the lung | Combined nivo and ipi | SD | 12 | 12 | B2M frameshift mutation in both primary and metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, M.; Durhuus, J.A.; Nilbert, M.; Andersen, O.; Therkildsen, C. Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 329. https://doi.org/10.3390/jcm12010329
Rasmussen M, Durhuus JA, Nilbert M, Andersen O, Therkildsen C. Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(1):329. https://doi.org/10.3390/jcm12010329
Chicago/Turabian StyleRasmussen, Maria, Jon Ambæk Durhuus, Mef Nilbert, Ove Andersen, and Christina Therkildsen. 2023. "Response to Immune Checkpoint Inhibitors Is Affected by Deregulations in the Antigen Presentation Machinery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 1: 329. https://doi.org/10.3390/jcm12010329