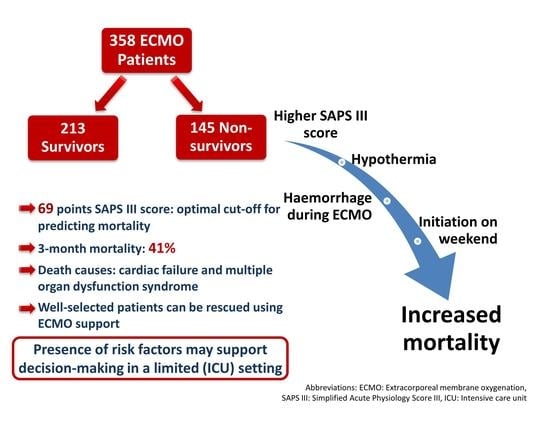
ECMO Predictors of Mortality: A 10-Year Referral Centre Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. ECMO Support and Anticoagulation
2.3. Data Acquisition
2.4. Outcomes
2.5. Statistical Analyses
3. Results
3.1. Patient and ECMO Characteristics
3.2. Outcomes
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.D.; O’Brien, T.G.; Murray, J.J.; Dontigny, L.; Bramson, M.L.; Osborn, J.J.; Gerbode, F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N. Engl. J. Med. 1972, 286, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, P.J.; Ball, C.M. The early history of extracorporeal membrane oxygenation. Anaesth. Intensiv. Care 2018, 46, 555–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasseur, A.; Scolletta, S.; Lorusso, R.; Taccone, F.S. Hybrid extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, S707–S715. [Google Scholar] [CrossRef] [PubMed]
- Bougouin, W.; Dumas, F.; Lamhaut, L.; Marijon, E.; Carli, P.; Combes, A.; Pirracchio, R.; Aissaoui, N.; Karam, N.; Deye, N.; et al. Extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: A registry study. Eur. Hear. J. 2019, 41, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Hifumi, T.; Sakamoto, T.; Kuroda, Y. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest in adult patients. J. Am. Hear. Assoc. 2020, 9, e015291. [Google Scholar] [CrossRef]
- Guidelines for Adult Respiratory Failure-Version 1.4. Available online: https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf (accessed on 15 December 2021).
- Brogan, T.V.; Lequier, L.; Lorusso, R.; MacLaren, G.; Peek, G.J. Extracorporeal Life Support: The ELSO Red Book, 5th ed.; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2017; p. 831. [Google Scholar]
- Biancari, F.; Mariscalco, G.; Dalén, M.; Settembre, N.; Welp, H.; Perrotti, A.; Wiebe, K.; Leo, E.; Loforte, A.; Chocron, S.; et al. Six-month survival after extracorporeal membrane oxygenation for severe COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1999–2006. [Google Scholar] [CrossRef]
- Ramanathan, K.; Shekar, K.; Ling, R.R.; Barbaro, R.P.; Wong, S.N.; Tan, C.S.; Rochwerg, B.; Fernando, S.M.; Takeda, S.; MacLaren, G.; et al. Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit. Care 2021, 25, 211. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. Extracorporeal Life Support Registry Report, International Summary; Extracorporeal Life Support Organization: Ann Arbor, MI, USA, 2020. [Google Scholar]
- Thompson, B.T.; Chambers, R.; Liu, K. Acute respiratory distress syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Combes, A.; Pesenti, A.; Ranieri, V.M. Fifty years of research in ARDS. Is extracorporeal circulation the future of acute respiratory distress syndrome management? Am. J. Respir. Crit. Care Med. 2017, 195, 1161–1170. [Google Scholar] [CrossRef]
- Schmidt, M.; Zogheib, E.; Rozé, H.; Repesse, X.; Lebreton, G.; Luyt, C.-E.; Trouillet, J.-L.; Bréchot, N.; Nieszkowska, A.; Dupont, H.; et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1704–1713. [Google Scholar] [CrossRef]
- Zavalichi, M.A.; Nistor, I.; Nedelcu, A.-E.; Zavalichi, S.D.; Georgescu, C.M.A.; Stătescu, C.; Covic, A. Extracorporeal membrane oxygenation in cardiogenic shock due to acute myocardial infarction: A systematic review. BioMed Res. Int. 2020, 2020, 6126534. [Google Scholar] [CrossRef] [PubMed]
- El Sibai, R.; Bachir, R.; El Sayed, M. Outcomes in cardiogenic shock patients with extracorporeal membrane oxygenation use: A matched cohort study in hospitals across the United States. BioMed Res. Int. 2018, 2018, 2428648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, L.A.; Teboul, J.-L. Mechanical circulatory support devices for cardiogenic shock: State of the art. Crit. Care 2019, 23, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Pham, T.; Combes, A.; Rozé, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)–induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef]
- Davies, A.H.; Jones, D.; Bailey, M.; Beca, J.; Bellomo, R.; Blackwell, N.; Forrest, P.; Gattas, D.; Granger, E.; Jackson, A.; et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA J. Am. Med. Assoc. 2009, 302, 1888–1895. [Google Scholar] [CrossRef] [Green Version]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Lequier, L.; Annich, G.; Al-Ibrahim, O.; Bembea, M.; Brodie, D.; Brogan, T.; Buckvold, S.; Chicoine, L.; Conrad, S.; Cooper, D.; et al. ELSO Anticoagulation Guideline; The Extracorporeal Life Support Organization (ELSO): Ann Arbor, MI, USA, 2014. [Google Scholar]
- Sklar, M.C.; Sy, E.; Lequier, L.; Fan, E.; Kanji, H.D. Anticoagulation practices during venovenous extracorporeal membrane oxygenation for respiratory failure. A systematic review. Ann. Am. Thorac. Soc. 2016, 13, 2242–2250. [Google Scholar] [CrossRef]
- Gray, B.W.; Haft, J.W.; Hirsch, J.C.; Annich, G.M.; Hirschl, R.B.; Bartlett, R.H. Extracorporeal life support: Experience with 2000 patients. ASAIO J. 2015, 61, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Mateo-Sidron, J.A.R.; Usman, A.; Fan, E. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef]
- Shekar, K.; Badulak, J.; Peek, G.; Boeken, U.; Dalton, H.J.; Arora, L.; Zakhary, B.; Ramanathan, K.; Starr, J.; Akkanti, B.; et al. Extracorporeal Life Support Organization coronavirus disease 2019 interim guidelines: A consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020, 66, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Life Expectancy at Birth-Austria. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=AT&most_recent_value_desc=true (accessed on 23 December 2021).
- Allokationsethische Orientierungshilfe für den Einsatz Knapper Intensivmedizinischer Ressourcen. 2020. Available online: https://www.intensivmedizin.at/sites/default/files/konsensus_fasim_allokation_intensiv_finale_fassung_text_11.11.2020_korr_neu_fin.pdf (accessed on 10 December 2021).
- Alonso-Fernandez-Gatta, M.; Merchan-Gomez, S.; Toranzo-Nieto, I.; Gonzalez-Cebrian, M.; Diego-Nieto, A.; Barrio, A.; Martin-Herrero, F.; Sanchez, P.L. Short-term mechanical circulatory support in elderly patients. Artif. Organs 2021. [Google Scholar] [CrossRef]
- Choi, M.J.; Ha, S.O.; Kim, H.S.; Park, S.; Han, S.J.; Lee, S.H. The simplified acute physiology score II as a predictor of mortality in patients who underwent extracorporeal membrane oxygenation for septic shock. Ann. Thorac. Surg. 2017, 103, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.I.; Lee, H.S.; Kim, H.S.; Ha, S.O.; Lee, W.Y.; Park, S.J.; Lee, S.H.; Lee, T.H.; Seo, J.Y.; Choi, H.H.; et al. The pre-ECMO simplified acute physiology score II as a predictor for mortality in patients with initiation ECMO support at the emergency department for acute circulatory and/or respiratory failure: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, K.M.; Au, S.Y.; Ng, G.W.Y.; Leung, A.K.H. Bleeding, thrombosis and transfusion in patients on ECMO: A retrospective study in a tertiary center in Hong Kong. Int. J. Artif. Organs 2021, 44, 420–425. [Google Scholar] [CrossRef]
- Cheng, A.; Sun, H.-Y.; Tsai, M.-S.; Ko, W.-J.; Tsai, P.-R.; Hu, F.-C.; Chen, Y.-C.; Chang, S.-C. Predictors of survival in adults undergoing extracorporeal membrane oxygenation with severe infections. J. Thorac. Cardiovasc. Surg. 2016, 152, 1526–1536.e1. [Google Scholar] [CrossRef] [Green Version]
- Bjertnæs, L.J.; Hindberg, K.; Næsheim, T.O.; Suborov, E.V.; Reierth, E.; Kirov, M.Y.; Lebedinskii, K.M.; Tveita, T. Rewarming from hypothermic cardiac arrest applying extracorporeal life support: A systematic review and meta-analysis. Front. Med. 2021, 8, 641633. [Google Scholar] [CrossRef]
- Aylin, P.; Alexandrescu, R.; Jen, M.H.; Mayer, E.; Bottle, A. Day of week of procedure and 30 day mortality for elective surgery: Retrospective analysis of hospital episode statistics. BMJ 2013, 346, f2424. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.S.; Wills, R.-A.; Bowman, R.V.; Zimmerman, P.V.; Fong, K.; Coory, M.D.; Yang, I. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern. Med. J. 2010, 40, 777–783. [Google Scholar] [CrossRef]
- Aubron, C.; Depuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensiv. Care 2016, 6, 97. [Google Scholar] [CrossRef] [Green Version]
- Arachchillage, D.J.; Rajakaruna, I.; Scott, I.; Gaspar, M.; Odho, Z.; Banya, W.; Vlachou, A.; Isgro, G.; Cagova, L.; Wade, J.; et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br. J. Haematol. 2021, 196, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Bréchot, N.; Hajage, D.; Kimmoun, A.; Demiselle, J.; Agerstrand, C.; Montero, S.; Schmidt, M.; Luyt, C.-E.; Lebreton, G.; Hékimian, G.; et al. Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: A retrospective, multicentre, international cohort study. Lancet 2020, 396, 545–552. [Google Scholar] [CrossRef]
- Rajsic, S.; Breitkopf, R.; Bachler, M.; Treml, B. Diagnostic Modalities in Critical Care: Point-of-Care Approach. Diagnostics 2021, 11, 2202. [Google Scholar] [CrossRef] [PubMed]



| Patient Characteristics * | All Patients (n = 358) | Survivors (n = 213) | Nonsurvivors (n = 145) | p-Value | Missing Data (n/Total) |
|---|---|---|---|---|---|
| Age (years) | 58.6 ± 15.9 | 57.6 ± 15.9 | 60.2 ± 15.9 | 0.135 | 0/358 |
| <30 31–45 46–60 61–70 >71 | 25 (7.0) 40 (11.2) 106 (29.6) 95 (26.5) 92 (25.7) | 17 (8.0) 24 (11.3) 71 (33.3) 50 (23.5) 51 (23.9) | 8 (5.5) 16 (11.0) 35 (24.1) 45 (31.0) 41 (28.3) | 0.224 | 0/358 |
| Male sex | 251 (70.1) | 151 (70.9) | 100 (69.0) | 0.725 | 0/358 |
| Height (cm) | 172 ± 10.0 | 173 ± 9.2 | 171 ± 11.1 | 0.133 | 10/358 |
| Weight (kg) | 80.8 ± 17.7 | 81.1 ± 17.6 | 80.4 ± 17.8 | 0.731 | 8/358 |
| Body mass index (kg/m2) | 27.2 ± 5.2 | 27.0 ± 5.1 | 27.5 ± 5.4 | 0.448 | 10/358 |
| SAPS III score | 67 (28–117) | 62 (28–112) | 73 (31–117) | <0.001 | 1/358 |
| SAPS III score predicted mortality (%) | 50 (1–96) | 40 (1–95) | 62 (2–96) | <0.001 | 1/358 |
| SOFA score | 12 (2–21) | 11 (3–21) | 12 (2–21) | 0.003 | 0/358 |
| SOFA respiratory | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.451 | |
| SOFA coagulation | 1 (0–4) | 1 (0–4) | 1 (0–4) | 0.722 | |
| SOFA liver | 1 (0–4) | 1 (1–3) | 0 (0–4) | 0.383 | |
| SOFA cardiovascular | 4 (0–4), mean 3.5 | 4 (0–4), mean 3.4 | 4 (0–4), mean 3.7 | <0.001 | |
| SOFA neurology | 4 (0–4), mean 2.9 | 4 (0–4), mean 2.7 | 4 (0–4), mean 3.2 | 0.002 | |
| SOFA renal | 1 (0–4) | 1 (0–4) | 1 (0–4) | 0.070 | |
| CPR before ECMO initiation | 65 (18.2) | 31 (14.6) | 34 (23.4) | 0.032 | 0/358 |
| Length of ICU stay (days) | 18 (1–170) | 21 (6–170) | 10 (1–79) | <0.001 | 0/358 |
| ICU admission reason | 0/358 | ||||
| Respiratory failure | 81 (22.6) | 52 (24.4) | 29 (20.0) | 0.014 | |
| Cardiac nonsurgical | 187 (52.2) | 107 (50.2) | 80 (55.2) | ||
| Cardiac surgery | 75 (20.9) | 50 (23.5) | 25 (17.2) | ||
| Trauma | 3 (0.8) | 2 (0.9) | 1 (0.7) | ||
| Hypothermia | 12 (3.4) | 2 (0.9) | 10 (6.9) | ||
| ICU department | 0/358 | ||||
| ICU 1 | 193 (53.9) | 122 (57.3) | 71 (49.0) | 0.121 | |
| ICU 2 | 165 (46.1) | 91 (42.7) | 74 (51.0) | ||
| Mortality-related outcomes | 0/358 | ||||
| Admission to death (days) | 10 (1–88) | - | - | ||
| ECMO initiation to death (days) | 9 (1–87) | - | - | ||
| Survived beyond ECMO support | 277 (77.4) | - | - | ||
| Survived beyond ICU | 227 (63.4) | - | - | ||
| Discharged alive | 216 (60.3) | - | - | ||
| Survived beyond one year | 206 (57.5) | - | - |
| Clinical Characteristics * | All Patients (n = 358) | Survivors (n = 213) | Nonsurvivors (n = 145) | p-Value | Missing Data (n/Total) |
|---|---|---|---|---|---|
| ECMO support indications | 0/358 | ||||
| Cardiogenic shock | 258 (72.1) | 153 (71.8) | 105 (72.4) | ||
| Respiratory failure | 88 (24.6) | 58 (27.2) | 30 (20.7) | 0.014 | |
| Hypothermia | 12 (3.4) | 2 (0.9) | 10 (6.9) | ||
| Type of ECMO support | 0/358 | ||||
| Venoarterial | 284 (79.3) | 165 (77.5) | 119 (82.1) | 0.163 | |
| Venovenous | 74 (20.7) | 48 (22.5) | 26 (17.9) | ||
| ECMO-related clinical course | 0/358 | ||||
| ECMO support duration (days) | 6 (1–36) | 6 (1–36) | 6 (1–36) | 0.315 | |
| ECMO support duration < 7 days | 238 (66.5) | 147 (69.0) | 91 (62.1) | 0.218 | |
| Time from admission to ECMO initiation (days) | 0 (0–36) | 0 (0–9) | 0 (0–36) | 0.449 | |
| Day of ECMO support initiation | 0/358 | ||||
| Week day | 289 (80.7) | 181 (85.0) | 108 (74.5) | 0.013 | |
| Weekends | 69 (19.3) | 32 (15.0) | 37 (25.5) | ||
| Anticoagulation during ECMO support | 1/358 | ||||
| UFH | 278 (77.9) | 176 (82.6) | 102 (70.8) | 0.015 | |
| Argatroban | 32 (9.0) | 19 (8.9) | 13 (9.0) | ||
| Epoprostenol | 1 (0.3) | 0 (0.0) | 1 (0.7) | ||
| Argatroban and epoprostenol | 5 (1.4) | 2 (0.9) | 3 (2.1) | ||
| None | 41 (11.5) | 16 (7.5) | 25 (17.4) | ||
| Reason for ECMO support termination | 0/358 | ||||
| Improvement (weaned) | 252 (70.4) | 195 (91.5) | 57 (39.3) | <0.001 | |
| Bridge to other assistance (heart transplant or VAD) | 18 (5.0) | 16 (7.5) | 2 (1.4) | ||
| Haemorrhage | 7 (2.0) | 2 (0.9) | 5 (3.5) | ||
| Death | 81 (22.6) | - | 81 (55.9) | ||
| Complications * | All Patients (n = 358) | Survivors (n = 213) | Nonsurvivors (n = 145) | p-Value | Missing Data (n/Total) |
|---|---|---|---|---|---|
| Haemorrhage | 160 (44.7) | 79 (37.1) | 81 (55.9) | <0.001 | 0/358 |
| Major haemorrhage | 96 (26.8) | 43 (20.2) | 53 (36.6) | 0.001 | 0/358 |
| Minor haemorrhage | 64 (17.9) | 36 (16.9) | 28 (19.3) | 0.559 | 0/358 |
| Day of haemorrhage | 1 (1–14) | 1 (1–14) | 1 (1–14) | 0.589 | 0/358 |
| Haemorrhage on the first ECMO support day | 86 (24.0) | 42 (19.7) | 44 (30.3) | 0.883 | 0/358 |
| Haemorrhage within first three days | 117 (32.7) | 56 (26.3) | 61 (42.1) | 0.533 | 0/358 |
| Coagulopathy | 46 (12.8) | 22 (10.3) | 24 (16.6) | 0.084 | 0/358 |
| Thrombosis | 82 (22.9) | 48 (22.5) | 34 (23.4) | 0.840 | 0/358 |
| Thrombosis venous | 56 (15.6) | 36 (16.9) | 20 (13.8) | 0.427 | 0/358 |
| Thrombosis arterial | 40 (11.2) | 19 (8.9) | 21 (14.5) | 0.101 | 0/358 |
| Sepsis | 71 (19.8) | 33 (15.5) | 38 (26.2) | 0.013 | 0/358 |
| Substitution of blood products during ECMO support | 26/358 | ||||
| Packed red blood cells (units) | 6 (0–60) | 5 (0–36) | 8 (0–60) | <0.001 | |
| Fresh-frozen plasma (units) | 0 (0–92) 3.4 ± 8.6 | 0 (0–40) 2.6 ± 6.5 | 0 (0–92) 4.5 ± 10.8 | 0.022 | |
| Platelets (units) | 1 (0–30) | 0 (0–30) | 1 (0–22) | 0.006 | |
| Fibrinogen (g) | 0 (0–26) 3.0 ± 4.9 | 0 (0–20) 2.2 ± 3.9 | 0 (0–26) 4.1 ± 5.9 | 0.003 | |
| Antithrombin (IU) | 0 (0–32,000) | 0 (0–32,000) | 0 (0–15,500) | 0.478 | |
| Prothrombin complex concentrate (IU) | 0 (0–7200) 436 ± 1034.2 | 0 (0–3600) 272 ± 700.2 | 0 (0–7200) 677 ± 1353.3 | 0.005 | |
| Factor XIII concentrate (IU) | 0 (0–10,000) | 0 (0–6250) | 0 (0–10,000) | 0.089 | |
| Desmopressin (µg) | 0 (0–30) | 0 (0–30) | 0 (0–30) | 0.773 | |
| Von Willebrand Factor (IU) | 0 (0–5000) 104 ± 548.9 | 0 (0–5000) 76 ± 537.3 | 0 (0–4000) 145 ± 564.9 | 0.021 | |
| Nondependent Variable | B-Coefficient | p-Value | HR | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| ECMO initiation on weekends | 0.471 | 0.016 | 1.60 | 1.09 | 2.35 |
| Resuscitation before ECMO | 0.189 | 0.352 | 1.21 | 0.81 | 1.81 |
| SAPS III score | 0.037 | <0.001 | 1.04 | 1.03 | 1.05 |
| ECMO indication respiratory failure (reference category) | |||||
| Cardiogenic shock | 0.410 | 0.066 | 1.51 | 0.97 | 2.33 |
| Hypothermia | 1.321 | <0.001 | 3.75 | 1.79 | 7.87 |
| Haemorrhage | 0.551 | 0.001 | 1.74 | 1.24 | 2.43 |
| Sepsis | 0.180 | 0.376 | 1.20 | 0.80 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treml, B.; Breitkopf, R.; Bukumirić, Z.; Bachler, M.; Boesch, J.; Rajsic, S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. J. Clin. Med. 2022, 11, 1224. https://doi.org/10.3390/jcm11051224
Treml B, Breitkopf R, Bukumirić Z, Bachler M, Boesch J, Rajsic S. ECMO Predictors of Mortality: A 10-Year Referral Centre Experience. Journal of Clinical Medicine. 2022; 11(5):1224. https://doi.org/10.3390/jcm11051224
Chicago/Turabian StyleTreml, Benedikt, Robert Breitkopf, Zoran Bukumirić, Mirjam Bachler, Johannes Boesch, and Sasa Rajsic. 2022. "ECMO Predictors of Mortality: A 10-Year Referral Centre Experience" Journal of Clinical Medicine 11, no. 5: 1224. https://doi.org/10.3390/jcm11051224