Risk of Pacing-Induced Cardiomyopathy in Patients with High-Degree Atrioventricular Block—Impact of Right Ventricular Lead Position Confirmed by Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Characteristics and Outcomes
2.3. Pacemaker Implantation and Interrogation
2.4. Cardiac Computed Tomography—Acquisition and Analysis
2.5. Echocardiography
2.6. Statistical Analysis
3. Results
3.1. Patient Selection
3.2. CT-Determined RV Lead Position
3.3. Clinical Characteristics in Patients with Septal and Non-Septal RV Lead Position
3.4. Outcomes and RV Lead Position
4. Discussion
4.1. Main Findings
4.2. The Importance of RV Lead Position—Fluoroscopy versus Cardiac CT
4.3. Risk of PICM—Comparison with Previous Studies
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and Predictors of Right Ventricular Pacing-Induced Cardiomyopathy in Patients with Complete Atrioventricular Block and Preserved Left Ventricular Systolic Function. Heart Rhythm 2016, 13, 2272–2278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tops, L.F.; Schalij, M.J.; Bax, J.J. The Effects of Right Ventricular Apical Pacing on Ventricular Function and Dyssynchrony. Implications for Therapy. J. Am. Coll. Cardiol. 2009, 54, 764–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillock, R.J.; Mond, H.G. Pacing the Right Ventricular Outflow Tract Septum: Time to Embrace the Future. Europace 2012, 14, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kaye, G.C.; Linker, N.J.; Marwick, T.H.; Pollock, L.; Graham, L.; Pouliot, E.; Poloniecki, J.; Gammage, M.; Kaye, G.C.; Martin, P.; et al. Effect of Right Ventricular Pacing Lead Site on Left Ventricular Function in Patients with High-Grade Atrioventricular Block: Results of the Protect-Pace Study. Eur. Heart J. 2015, 36, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenichini, G.; Sunthorn, H.; Fleury, E.; Foulkes, H.; Stettler, C.; Burri, H. Pacing of the Interventricular Septum versus the Right Ventricular Apex: A Prospective, Randomized Study. Eur. J. Intern. Med. 2012, 23, 621–627. [Google Scholar] [CrossRef]
- Molina, L.; Sutton, R.; Gandoy, W.; Reyes, N.; Lara, S.; Limón, F.; Gómez, S.; Orihuela, C.; Salame, L.; Moreno, G. Medium-Term Effects of Septal and Apical Pacing in Pacemaker-Dependent Patients: A Double-Blind Prospective Randomized Study. PACE—Pacing Clin. Electrophysiol. 2014, 37, 207–214. [Google Scholar] [CrossRef]
- Riahi, S.; Nielsen, J.C.; Hjortshoj, S.; Thomsen, P.E.; Hojberg, S.; Moller, M.; Dalsgaard, D.; Nielsen, T.; Asklund, M.; Friis, E.V.; et al. Heart Failure in Patients with Sick Sinus Syndrome Treated with Single Lead Atrial or Dual-Chamber Pacing: No Association with Pacing Mode or Right Ventricular Pacing Site. Europace 2012, 14, 1475–1482. [Google Scholar] [CrossRef]
- Sommer, A.; Kronborg, M.B.; Nørgaard, B.L.; Gerdes, C.; Mortensen, P.T.; Nielsen, J.C.; Norgaard, B.L.; Gerdes, C.; Mortensen, P.T.; Nielsen, J.C. Left and Right Ventricular Lead Positions Are Imprecisely Determined by Fluoroscopy in Cardiac Resynchronization Therapy: A Comparison with Cardiac Computed Tomography. Europace 2014, 16, 1334–1341. [Google Scholar] [CrossRef]
- Osmancik, P.; Stros, P.; Herman, D.; Curila, K.; Petr, R. The Insufficiency of Left Anterior Oblique and the Usefulness of Right Anterior Oblique Projection for Correct Localization of a Computed Tomography-Verified Right Ventricular Lead into the Midseptum. Circ. Arrhythmia Electrophysiol. 2013, 6, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Hattori, M.; Naruse, Y.; Oginosawa, Y.; Matsue, Y.; Hanaki, Y.; Kowase, S.; Kurosaki, K.; Mizukami, A.; Kohno, R.; Abe, H.; et al. Prognostic Impact of Lead Tip Position Confirmed via Computed Tomography in Patients with Right Ventricular Septal Pacing. Heart Rhythm 2019, 16, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Shimony, A.; Eisenberg, M.J.; Filion, K.B.; Amit, G. Beneficial Effects of Right Ventricular Non-Apical vs. Apical Pacing: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Europace 2012, 14, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Furuya-Kanamori, L.; Kaye, G.; Clark, J.; Doi, S.A.R. The Effect of Right Ventricular Apical and Nonapical Pacing on the Short- and Long-Term Changes in Left Ventricular Ejection Fraction: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Pacing Clin. Electrophysiol. 2015, 38, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Kaye, G.; Ng, J.Y.; Ahmed, S.; Valencia, D.; Harrop, D.; Ng, A.C.T. The Prevalence of Pacing-Induced Cardiomyopathy (PICM) in Patients With Long Term Right Ventricular Pacing—Is It a Matter Of Definition? Heart. Lung Circ. 2019, 28, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Epstein, A.E.; Verdino, R.J.; Lin, D.; Goldberg, L.R.; Marchlinski, F.E.; Frankel, D.S. Incidence and Predictors of Right Ventricular Pacing-Induced Cardiomyopathy. Heart Rhythm 2014, 11, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Abdin, A.; Yalin, K.; Zink, M.D.; Napp, A.; Gramlich, M.; Marx, N.; Schuett, K. Incidence and Predictors of Pacemaker Induced Cardiomyopathy: A Single-Center Experience. J. Electrocardiol. 2019, 57, 31–34. [Google Scholar] [CrossRef]
- Dor, O.; Haim, M.; Barrett, O.; Novack, V.; Konstantino, Y. Incidence and Clinical Outcomes of Pacing Induced Cardiomyopathy in Patients With Normal Left Ventricular Systolic Function and Atrioventricular Block. Am. J. Cardiol. 2020, 128, 174–180. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chen, H.; Siu, C.W.; Yiu, K.H.; Chan, W.S.; Lee, K.L.; Chan, H.W.; Lee, S.W.; Fu, G.S.; Lau, C.P.; et al. New-Onset Heart Failure after Permanent Right Ventricular Apical Pacing in Patients with Acquired High-Grade Atrioventricular Block and Normal Left Ventricular Function. J. Cardiovasc. Electrophysiol. 2008, 19, 136–141. [Google Scholar] [CrossRef]
- Cho, S.W.; Gwag, H.B.; Hwang, J.K.; Chun, K.J.; Park, K.M.; On, Y.K.; Kim, J.S.; Park, S.J. Clinical Features, Predictors, and Long-Term Prognosis of Pacing-Induced Cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 643–651. [Google Scholar] [CrossRef]
- Kaye, G. The Desire for Physiological Pacing: Are We There Yet? J. Cardiovasc. Electrophysiol. 2019, 30, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]


| Variables | Non-Septal (n = 105) | Septal (n = 48) | p-value | All (n = 153) |
| Age (y) | 72.9 (64.6–77.7) | 72.0 (66.3–75.8) | 0.92 | 72.1 (64.7–76.9) |
| Male sex | 72 (68.6) | 31 (64.6) | 0.71 | 103 (67.3) |
| LVEF (%) | 60.2 ± 3.5 | 60.6 ± 3.8 | 0.51 | 60.3 ± 3.6 |
| QRS duration (ms) | 119 ± 30 | 120 ± 29 | 0.74 | 119 ± 30 |
| Comorbidity | ||||
| Ischemic heart disease | 5 (4.8) | 7 (14.6) | 0.05 | 12 (7.8) |
| Valvular heart disease | 7 (6.7) | 5 (10.4) | 0.52 | 12 (7.8) |
| Atrial fibrillation | 11 (10.5) | 4 (8.3) | 0.78 | 15 (9.8) |
| Hypertension | 70 (66.7) | 36 (75.0) | 0.35 | 106 (69.3) |
| Diabetes | 24 (22.9) | 11 (22.9) | 1.00 | 35 (22.9) |
| History of smoking | 41 (39.1) | 24 (50.0) | 0.22 | 65 (42.5) |
| eGFR (mL/min/1.73 m2) | 75.3 ± 16.2 | 73.8 ± 13.6 | 0.57 | 74.8 ± 15.4 |
| Medical therapy | ||||
| RAS-acting agents | 52 (49.5) | 30 (62.5) | 0.16 | 82 (53.6) |
| Betablocker | 20 (19.1) | 9 (18.8) | 1.00 | 29 (19.0) |
| Loop diuretics | 12 (11.4) | 3 (6.3) | 0.39 | 15 (9.8) |
| Aldosterone antagonist | 5 (5.8) | 3 (6.3) | 0.71 | 8 (5.2) |
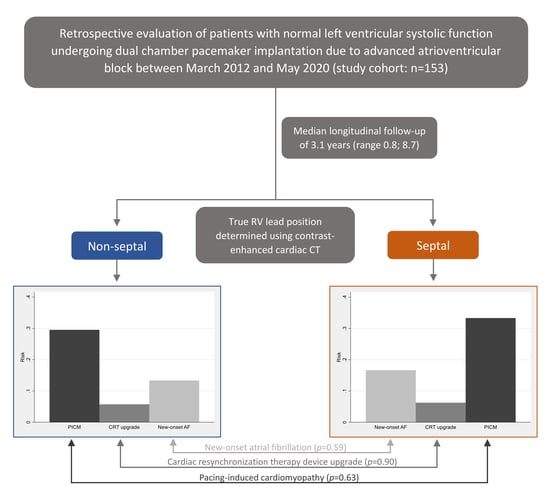
| Outcomes | All (n = 153) | Non-Septal (n = 105) | Septal (n = 48) | RR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| PICM | 47 (30.7) | 31 (29.5) | 16 (33.3) | 0.89 | 0.54; 1.46 | 0.63 |
| CRT upgrade | 9 (5.9) | 6 (5.7) | 3 (6.3) | 0.91 | 0.23; 3.52 | 0.90 |
| New-onset AF | 22 (14.4) | 14 (13.3) | 8 (16.7) | 0.80 | 0.36; 1.78 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruelund, P.Z.; Sommer, A.; Frøkjær, J.B.; Lundbye-Christensen, S.; Zaremba, T.; Søgaard, P.; Graff, C.; Vraa, S.; Mahalingasivam, A.A.; Thøgersen, A.M.; et al. Risk of Pacing-Induced Cardiomyopathy in Patients with High-Degree Atrioventricular Block—Impact of Right Ventricular Lead Position Confirmed by Computed Tomography. J. Clin. Med. 2022, 11, 7228. https://doi.org/10.3390/jcm11237228
Fruelund PZ, Sommer A, Frøkjær JB, Lundbye-Christensen S, Zaremba T, Søgaard P, Graff C, Vraa S, Mahalingasivam AA, Thøgersen AM, et al. Risk of Pacing-Induced Cardiomyopathy in Patients with High-Degree Atrioventricular Block—Impact of Right Ventricular Lead Position Confirmed by Computed Tomography. Journal of Clinical Medicine. 2022; 11(23):7228. https://doi.org/10.3390/jcm11237228
Chicago/Turabian StyleFruelund, Patricia Zerlang, Anders Sommer, Jens Brøndum Frøkjær, Søren Lundbye-Christensen, Tomas Zaremba, Peter Søgaard, Claus Graff, Søren Vraa, Aksayan Arunanthy Mahalingasivam, Anna Margrethe Thøgersen, and et al. 2022. "Risk of Pacing-Induced Cardiomyopathy in Patients with High-Degree Atrioventricular Block—Impact of Right Ventricular Lead Position Confirmed by Computed Tomography" Journal of Clinical Medicine 11, no. 23: 7228. https://doi.org/10.3390/jcm11237228