Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients
Abstract
:1. Introduction
2. Material and Methods
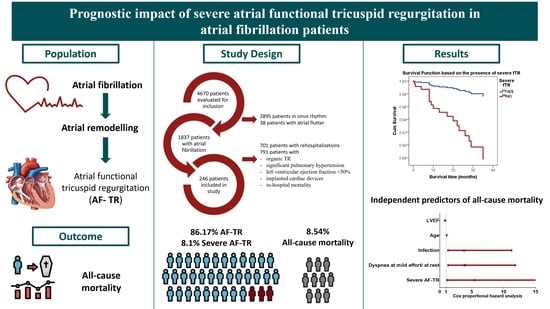
2.1. Population
2.2. Definitions
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Patients with Severe AF-TR
3.2. Severe AF-TR and Mortality
4. Discussion
4.1. Atrial Functional Tricuspid Regurgitation
4.2. Severe AF-TR and Mortality of AF Patients
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Vinter, N.; Huang, Q.; Fenger-Grøn, M.; Frost, L.; Benjamin, E.; Trinquart, L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): Community based cohort study. BMJ 2020, 370, m2724. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: Results from the biomarcare consortium (Biomarker for cardiovascular risk assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Staerk, L.; Wang, B.; Preis, S.R.; Larson, M.G.; Lubitz, S.A.; Ellinor, P.T.; McManus, D.D.; Ko, D.; Weng, L.-C.; Lunetta, K.L.; et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: Cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018, 361, k1453. [Google Scholar] [CrossRef] [Green Version]
- Vîjan, A.E.; Daha, I.C.; Delcea, C.; Dan, G.-A. Determinants of prolonged length of hospital stay of patients with atrial fibrillation. J. Clin. Med. 2021, 10, 3715. [Google Scholar] [CrossRef]
- Fauchier, L.; Villejoubert, O.; Clementy, N.; Bernard, A.; Pierre, B.; Angoulvant, D.; Ivanes, F.; Babuty, D.; Lip, G.Y.H. Causes of Death and Influencing Factors in Patients with Atrial Fibrillation. Am. J. Med. 2016, 129, 1278–1287. [Google Scholar] [CrossRef]
- Delgado, V.; Bax, J.J. Atrial Functional Mitral Regurgitation: From Mitral Annulus Dilatation to Insufficient Leaflet Remodeling. Circ. Cardiovasc. Imaging 2017, 10, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Utsunomiya, H.; Itabashi, Y.; Mihara, H.; Berdejo, J.; Kobayashi, S.; Siegel, R.J.; Shiota, T. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ. Cardiovasc. Imaging 2017, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Topilsky, Y.; Maltais, S.; Medina Inojosa, J.; Oguz, D.; Michelena, H.; Maalouf, J.; Mahoney, D.W.; Enriquez-Sarano, M. Burden of Tricuspid Regurgitation in Patients Diagnosed in the Community Setting. JACC Cardiovasc. Imaging 2019, 12, 433–442. [Google Scholar] [CrossRef]
- Topilsky, Y.; Khanna, A.; Le Toumeau, T.; Park, S.; Michelena, H.; Suri, R.; Mahoney, D.W.; Enriquez-Sarano, M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 314–323. [Google Scholar] [CrossRef]
- Spinner, E.M.; Shannon, P.; Buice, D.; Jimenez, J.H.; Veledar, E.; del Nido, P.J.; Adams, D.H.; Yoganathan, A.P. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 2011, 124, 920–929. [Google Scholar] [CrossRef] [Green Version]
- Najib, M.Q.; Vinales, K.L.; Vittala, S.S.; Challa, S.; Lee, H.R.; Chaliki, H.P. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Muraru, D.; Guta, A.C.; Ochoa-Jimenez, R.C.; Bartos, D.; Aruta, P.; Mihaila, S.; Popescu, B.A.; Iliceto, S.; Basso, C.; Badano, L.P. Functional Regurgitation of Atrioventricular Valves and Atrial Fibrillation: An Elusive Pathophysiological Link Deserving Further Attention. J. Am. Soc. Echocardiogr. 2020, 33, 42–53. [Google Scholar] [CrossRef]
- Florescu, D.R.; Muraru, D.; Volpato, V.; Gavazzoni, M.; Caravita, S.; Tomaselli, M.; Ciampi, P.; Florescu, C.; Bălșeanu, T.A.; Parati, G.; et al. Atrial Functional Tricuspid Regurgitation as a Distinct Pathophysiological and Clinical Entity: No Idiopathic Tricuspid Regurgitation Anymore. J. Clin. Med. 2022, 11, 382. [Google Scholar] [CrossRef]
- Muraru, D.; Parati, G.; Badano, L.P. The tale of functional tricuspid regurgitation: When atrial fibrillation is the villain. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1079–1081. [Google Scholar] [CrossRef]
- Guta, A.C.; Badano, L.P.; Tomaselli, M.; Mihalcea, D.; Bartos, D.; Parati, G.; Muraru, D. The Pathophysiological Link between Right Atrial Remodeling and Functional Tricuspid Regurgitation in Patients with Atrial Fibrillation: A Three-Dimensional Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 585–594.e1. [Google Scholar] [CrossRef]
- Matta, M.; Layoun, H.; Abou Hassan, O.K.; Rodriguez, L.; Schoenhagen, P.; Kanj, M.; Griffin, B.P.; Kapadia, S.R.; Harb, S.C. Mechanistic Insights Into Significant Atrial Functional Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 2049–2050. [Google Scholar] [CrossRef]
- Abe, Y.; Akamatsu, K.; Ito, K.; Matsumura, Y.; Shimeno, K.; Naruko, T.; Takahashi, Y.; Shibata, T.; Yoshiyama, M. Prevalence and Prognostic Significance of Functional Mitral and Tricuspid Regurgitation Despite Preserved Left Ventricular Ejection Fraction in Atrial Fibrillation Patients. Circ. J. 2018, 82, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Nath, J.; Foster, E.; Heidenreich, P.A. Impact of Tricuspid Regurgitation on Long-Term Survival. J. Am. Coll. Cardiol. 2004, 43, 405–409. [Google Scholar] [CrossRef]
- Topilsky, Y.; Nkomo, V.T.; Vatury, O.; Michelena, H.I.; Letourneau, T.; Suri, R.M.; Pislaru, S.; Park, S.; Mahoney, D.W.; Biner, S.; et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc. Imaging 2014, 7, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; de Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2786. [Google Scholar] [CrossRef] [PubMed]
- Prapan, N.; Ratanasit, N.; Karaketklang, K. Significant functional tricuspid regurgitation portends poor outcomes in patients with atrial fibrillation and preserved left ventricular ejection fraction. BMC Cardiovasc. Disord. 2020, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Dietz, M.F.; Goedemans, L.; Vo, N.M.; Prihadi, E.A.; van der Bijl, P.; Gersh, B.J.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Significant Isolated Tricuspid Regurgitation in Patients with Atrial Fibrillation Without Left-Sided Heart Disease or Pulmonary Hypertension. Am. J. Cardiol. 2020, 135, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; de Ferrari, G.M.; Knuuti, J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Prognostic Implications of a Novel Algorithm to Grade Secondary Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 1085–1095. [Google Scholar] [CrossRef]
- Samaras, A.; Vrana, E.; Kartas, A.; Moysidis, D.V.; Papazoglou, A.S.; Doundoulakis, I.; Fotos, G.; Rampidis, G.; Tsalikakis, D.G.; Efthimiadis, G.; et al. Prognostic implications of valvular heart disease in patients with non-valvular atrial fibrillation. BMC Cardiovasc. Disord. 2021, 21, 453. [Google Scholar] [CrossRef]
- Muraru, D.; Caravita, S.; Guta, A.C.; Mihalcea, D.; Branzi, G.; Parati, G.; Badano, L.P. Functional Tricuspid Regurgitation and Atrial Fibrillation: Which Comes First, the Chicken or the Egg? CASE Cardiovasc. Imaging Case Rep. 2020, 4, 458. [Google Scholar] [CrossRef]
- Mutlak, D.; Lessick, J.; Reisner, S.A.; Aronson, D.; Dabbah, S.; Agmon, Y. Echocardiography-based Spectrum of Severe Tricuspid Regurgitation: The Frequency of Apparently Idiopathic Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2007, 20, 405–408. [Google Scholar] [CrossRef]
- Mutlak, D.; Khalil, J.; Lessick, J.; Kehat, I.; Agmon, Y.; Aronson, D. Risk Factors for the Development of Functional Tricuspid Regurgitation and Their Population-Attributable Fractions. JACC Cardiovasc. Imaging 2020, 13, 1643–1651. [Google Scholar] [CrossRef]
- Alonso, A.; Almuwaqqat, Z.; Chamberlain, A. Mortality in atrial fibrillation. Is it changing? Trends Cardiovasc. Med. 2021, 31, 469–473. [Google Scholar] [CrossRef]
- Itakura, K.; Hidaka, T.; Nakano, Y.; Utsunomiya, H.; Kinoshita, M.; Susawa, H.; Harada, Y.; Izumi, K.; Kihara, Y. Successful catheter ablation of persistent atrial fibrillation is associated with improvement in functional tricuspid regurgitation and right heart reverse remodeling. Heart Vessels 2020, 35, 842–851. [Google Scholar] [CrossRef]
- Masuda, M.; Sekiya, K.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; et al. Influence of catheter ablation for atrial fibrillation on atrial and ventricular functional mitral regurgitation. ESC Heart Fail. 2022, 9, 1901–1913. [Google Scholar] [CrossRef]
- He, S.; Jimenez, J.; He, Z.; Yoganathan, A.P. Mitral leaflet geometry perturbations with papillary muscle displacement and annular dilatation: An in-vitro study of ischemic mitral regurgitation. J. Heart Valve Dis. 2003, 12, 300–307. [Google Scholar]


| Total N = 246 | Non-Severe TR * N = 226 | Severe TR ** N = 20 | p Value for Comparison of * and ** | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 71.5 ± 9.4 | 71 ± 9.4 | 77 ± 7.7 | 0.005 |
| Women | 155 (63.0%) | 140 (62.0%) | 15 (75.0%) | 0.25 |
| Heart failure characteristics | ||||
| HF | 183 (74.4%) | 165 (73.0%) | 18 (90%) | 0.09 |
| ADHF | 65 (26.4%) | 55 (24.3%) | 10 (50%) | 0.01 |
| NYHA class 1–2 | 148 (60.2%) | 135 (59.7%) | 13 (65%) | 0.64 |
| NYHA class 3–4 | 35(14.2%) | 30 (13.3%) | 5 (25.0%) | 0.15 |
| AF characteristics | ||||
| Paroxysmal | 96 (39.0%) | 94 (41.6%) | 2 (10%) | 0.006 |
| Persistent | 71 (28.9%) | 66 (29.2%) | 5 (25%) | 0.7 |
| Permanent | 79 (32.1%) | 66 (29.2%) | 13 (65%) | 0.001 |
| CHA2DS2-VASc | 4 (3–5) | 4 (3–5) | 5 (4–5) | 0.04 |
| HAS-BLED | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.26 |
| Cardiovascular risk factors and comorbidities | ||||
| HTN | 215 (87.4%) | 198 (87.6%) | 17 (85%) | 0.74 |
| IHD | 66 (26.8%) | 64 (28.3%) | 2 (10%) | 0.08 |
| Prior MI | 15 (6.1%) | 14 (6.2%) | 1 (5%) | 0.83 |
| TIA/Stroke | 34 (13.8%) | 32 (14.2%) | 2 (10%) | 0.60 |
| Diabetes mellitus | 63 (25.6%) | 58 (25.7%) | 5 (25%) | 0.94 |
| Dyslipidemia | 197 (80.1%) | 186 (82.3%) | 11 (55%) | 0.003 |
| Obesity | 90 (36.6%) | 86 (38.0%) | 4 (20%) | 0.11 |
| Dementia | 9 (3.7%) | 9 (4%) | 0 (0%) | 0.36 |
| Infection | 31 (12.8%) | 28 (12.5%) | 3 (15.8%) | 0.68 |
| HR, bpm | 84.3 ± 23.1 | 84.2 ± 23.3 | 84.4 ± 20.7 | 0.75 |
| Echocardiographic characteristics | ||||
| LAD, mm | 43.5 ± 5.5 | 43.3 ± 5.5 | 46.1 ± 5.4 | 0.03 |
| LVEDD, mm | 48.1 ± 5.9 | 48.3 ± 5.8 | 45.5 ± 6 | 0.06 |
| LVESD, mm | 31.3 ± 5.4 | 31.5 ± 5.4 | 29.0 ± 5.4 | 0.06 |
| RAD, mm | 40.5 ± 6.4 | 39.8 ± 5.6 | 48.8 ± 10.2 | <0.001 |
| RVD, mm | 32.7 ± 5.7 | 32.1 ± 5.0 | 39.1 ± 8.6 | <0.001 |
| sPAP, mmHg | 28.8 ± 6.9 | 28.2 ± 6.8 | 35.7 ± 3.6 | <0.001 |
| LVEF, % | 55.9 ± 4.2 | 56.0 ± 4.3 | 54.7 ± 4.3 | 0.19 |
| Laboratory characteristics | ||||
| NT-proBNP, pg/mL | 1086 (539–1837) | 957 (486–1784) | 1686 (1300–3763) | 0.003 |
| eGFR, mL/min/1 | 75.7 (57.6–92.3) | 77.1 (59.9–93.0) | 55.34 (51.7–75.2) | 0.007 |
| HB, g/dL | 13.6 (12.4–14.6) | 13.6 (12.5–14.7) | 12.6 (11.3–14) | 0.30 |
| ADHF, acute decompensated heart failure; AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; LVEF, left ventricle ejection fraction; HB, hemoglobin; HF, Heart failure; HR, heart rate; HTN, arterial hypertension; IHD, ischemic heart disease; LAD, left atrium diameter; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; NYHA, New York Heart Association; RAD, right atrium diameter; RVD, right ventricular diameter; TIA, transient ischemic attack; TR, tricuspid regurgitation; sPAP, systolic pulmonary arterial pressure.* Patients with non-severe AF-TR; ** Patients with severe AF-TR | ||||
| Paroxysmal AF N = 96 | Persistent AF N = 71 | Permanent AF N = 79 | p for Trend | |
|---|---|---|---|---|
| Severe TR, n | 2 | 5 | 13 | 0.0006 |
| RAD, mm | 37.4 ± 4.5 | 41.2 ± 5.7 | 43.8 ± 7.3 | <0.001 |
| RVD, mm | 30.9 ± 4.7 | 33.3 ± 4.9 | 34.2 ± 6.8 | 0.0004 |
| sPAP, mmHg | 26.8 ± 7.1 | 28.9 ± 6.6 | 31.3 ± 6.3 | <0.001 |
| LVEF, % | 56.5 ± 4.4 | 56.0. ± 3.9 | 55.2 ± 4.5 | 0.13 |
| LAD, mm | 41.5 ± 5.6 | 43.7 ± 4.7 | 45.9 ± 5.2 | <0.001 |
| LVEDD, mm | 48.6 ± 6.4 | 47.2 ± 5.7 | 48.2 ± 5.2 | 0.32 |
| LVESD, mm | 31.9 ± 5.4 | 30.7 ± 5.6 | 31.1 ± 5.2 | 0.36 |
| OR (95% CI) | p Value | |
|---|---|---|
| ADHF at admission | 3.10 (1.2–7.8) | 0.01 |
| Permanent AF | 6.40 (1.5–28.3) | 0.005 |
| AUC (95% CI) | p Value | |
| Age | 0.70 (0.58–0.81) | 0.003 |
| LAD | 0.67 (0.50–0.80) | 0.015 |
| RAD | 0.81 (0.71–0.91) | <0.001 |
| RVD | 0.80 (0.70–0.90) | <0.001 |
| sPAP | 0.82 (0.75–0.90) | <0.001 |
| NT-proBNP | 0.76 (0.67–0.85) | 0.045 |
| eGFR | 0.68 (0.57–0.80) | 0.007 |
| CHA2DS2-VASc score | 0.63 (0.52–0.74) | 0.05 |
| HR (95% CI) | p Value | |
|---|---|---|
| RAD | 1.11 (1.03–1.20) | 0.005 |
| sPAP | 1.20 (1.03–1.38) | 0.015 |
| NTproBNP | 2.37 (1.10–5.10) | 0.026 |
| OR (95% CI) | p Value | |
|---|---|---|
| Severe AF-TR | 4.37 (1.41–13.57) | 0.005 |
| ADHF | 4.32 (1.72–10.83) | <0.001 |
| NYHA class III/IV | 5.74 (2.21–14.93) | <0.001 |
| TIA/Stroke | 2.81 (1.11–7.85) | 0.04 |
| Dementia | 6.08 (1.40–26.34) | 0.006 |
| Infection | 5.27 (2.02–14.21) | <0.001 |
| AUC (95% CI) | pValue | |
| Age | 0.77 (0.66–0.88) | <0.001 |
| RVD | 0.64 (0.50–0.78) | 0.05 |
| LVEF% | 0.69 (0.56–0.81) | 0.009 |
| NT-proBNP | 0.80 (0.71–0.90) | <0.001 |
| eGFR | 0.72 (0.61–0.84) | 0.01 |
| HB | 0.63 (0.50–0.76) | 0.05 |
| HR (95% CI) | p Value | |
|---|---|---|
| Severe AF-TR | 5.4 (1.1326.17) | 0.035 |
| Dyspnea at mild effort or at rest | 3.90 (1.29–11.82) | 0.016 |
| Infection | 3.79 (1.27–11.26) | 0.017 |
| Age | 1.07 (1.01–1.14) | 0.029 |
| LVEF | 0.82 (0.72–0.95) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vîjan, A.E.; Daha, I.C.; Delcea, C.; Bădilă, E.; Dan, G.-A. Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. J. Clin. Med. 2022, 11, 7145. https://doi.org/10.3390/jcm11237145
Vîjan AE, Daha IC, Delcea C, Bădilă E, Dan G-A. Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. Journal of Clinical Medicine. 2022; 11(23):7145. https://doi.org/10.3390/jcm11237145
Chicago/Turabian StyleVîjan, Ancuța Elena, Ioana Cristina Daha, Caterina Delcea, Elisabeta Bădilă, and Gheorghe-Andrei Dan. 2022. "Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients" Journal of Clinical Medicine 11, no. 23: 7145. https://doi.org/10.3390/jcm11237145