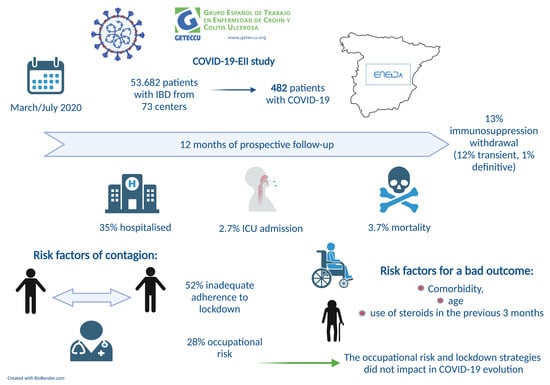
Nationwide COVID-19-EII Study: Incidence, Environmental Risk Factors and Long-Term Follow-Up of Patients with Inflammatory Bowel Disease and COVID-19 of the ENEIDA Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Data Collection
2.4. Definitions
2.5. Outcomes
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
3.1. Clinical Baseline Characteristics
3.2. Geografical Distribution of COVID-19 and Epidemiological Risk Factors of Exposure
3.3. COVID-19 Diagnosis and Treatment
3.4. Outcomes
3.5. Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John Hopkins University Coronavirus Resource Center. Coronavirus COVID-19 Global Cases. Available online: https://coronavirus.juy.edu/map.html (accessed on 21 August 2021).
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, M.F.; Dray-Spira, R.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabana, Y.; Rodríguez, L.; Lobatón, T.; Gordillo, J.; Montserrat, A.; Mena, R.; Beltrán, B.; Dotti, M.; Benitez, O.; Guardiola, J.; et al. Relevant Infections in Inflammatory Bowel Disease, and Their Relationship with Immunosuppressive Therapy and Their Effects on Disease Mortality. J. Crohn’s Colitis 2019, 13, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, A.; Navabi, S.; Williams, E.D.; Liu, G.; Kong, L.; Coates, M.D.; Clarke, K. Increased Risk of Influenza and Influenza-Related Complications among 140,480 Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Feuerstein, J.D.; Wang, A.Y.; Cohen, R.D. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease during the COVID-19 Pandemic: Expert Commentary. Gastroenterology 2020, 159, 350–357. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Jones, G.-R.; Lamb, C.A.; Appleby, R.; Arnott, I.; Beattie, R.M.; Bloom, S.; Brooks, A.J.; Cooney, R.; Dart, R.J.; et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020, 69, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C. 1st Interview COVID-19 ECCO Taskforce. 2020. Available online: https://ecco-ibd.eu/images/6_Publication/6_8_Surveys/1st_interview_COVID-19%20ECCOTaskforce_published.pdf (accessed on 2 August 2021).
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, but Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients with Inflammatory Bowel Diseases: Results from an International Registry. Gastroenterology 2020, 159, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.; Ungaro, R.; Colombel, J.; Kappelman, M. SECURE-IBD Database Public Data. Available online: https://covidibd.org/current-data/ (accessed on 24 August 2021).
- Aziz, M.; Fatima, R.; Haghbin, H.; Smith, W.L.; Nawras, A. The incidence and outcomes of COVID-19 in ibd patients: A rapid review and meta-Analysis. Inflamm. Bowel Dis. 2020, 26, E132–E133. [Google Scholar] [CrossRef]
- Mao, R.; Liang, J.; Shen, J.; Ghosh, S.; Zhu, L.-R.; Yang, H.; Wu, K.-C.; Chen, M.-H. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol. Hepatol. 2020, 5, 425–427. [Google Scholar] [CrossRef]
- Norsa, L.; Indriolo, A.; Sansotta, N.; Cosimo, P.; Greco, S.; D’Antiga, L. Uneventful course in IBD patients during SARS-CoV-2 outbreak in northern Italy. Gastroenterology 2020, 159, 371–372. [Google Scholar] [CrossRef]
- Allocca, M.; Fiorino, G.; Zallot, C.; Furfaro, F.; Radice, S.; Danese, S.; Peyrin-biroulet, L. Incidence and patterns of COVID-19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin. Gastroenterol. Hepatol. 2020, 18, 2134–2135. [Google Scholar] [CrossRef]
- Taxonera, C.; Sagastagoitia, I.; Alba, C.; Mañas, N.; Olivares, D.; Rey, E. 2019 novel coronavirus disease (COVID-19) in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2020, 52, 276–283. [Google Scholar] [CrossRef]
- Guerra, I.; Algaba, A.; Jiménez, L.; Aller, M.M.; Garza, D.; Bonillo, D.; Esteban, L.M.M.; Bermejo, F. Incidence, Clinical Characteristics, and Evolution of SARS-CoV-2 Infection in Patients with Inflammatory Bowel Disease: A Single-Center Study in Madrid, Spain. Inflamm. Bowel Dis. 2021, 27, 25–33. [Google Scholar] [CrossRef]
- Rodríguez-Lago, I.; Ramírez de la Piscina, P.; Elorza, A.; Merino, O.; Ortiz de Zárate, J.; Cabriada, J.L. Characteristics and Prognosis of Patients with Inflammatory Bowel Disease during the SARS-CoV-2 Pandemic in the Basque Country (Spain). Gastroenterology 2020, 159, 781–783. [Google Scholar] [CrossRef]
- Zabana, Y.; Panés, J.; Nos, P.; Gomollón, F.; Esteve, M.; García-Sánchez, V.; Gisbert, J.P.; Barreiro-de-Acosta, M.; Domènech, E. The ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU: Design, monitoring and functions. Gastroenterol. Hepatol. 2020, 43, 551–558. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005, 19 (Suppl. A), 5–36. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Gou, R.; Diao, Y.S.; Yin, Q.H.; Fan, W.X.; Liang, Y.P.; Chen, Y.; Wu, M.; Zang, L.; Li, L.; et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J. Zhejiang Univ. Sci. B 2014, 15, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Alves-Cabratosa, L.; Comas-Cufí, M.; Blanch, J.; Martí-Lluch, R.; Ponjoan, A.; Castro-Guardiola, A.; Hurtado-Ganoza, A.; Pérez-Jaén, A.; Rexach-Fumaña, M.; Faixedas-Brunsoms, D.; et al. Persons with SARS-CoV-2 during the First and Second Waves in Catalonia (Spain): A Retrospective Observational Study Using Daily Updated Data. JMIR Public Heal. Surveill. 2021, 8, e30006. [Google Scholar] [CrossRef] [PubMed]
- ISC; CNE. Informe Sobre la Situación de COVID-19 en España. Informe COVID-19 no12, 20 Marzo, 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/-COVID-19.-Informes-previos.aspx (accessed on 2 August 2021).
- CNE; ISC; ICI. COVID-19 en España. Available online: https://cnecovid.isciii.es/covid19/#provincias (accessed on 2 August 2021).
- Barreiro-de Acosta, M.; Gutiérrez, A.; Zabana, Y.; Beltrán, B.; Calvet, X.; Chaparro, M.; Domènech, E.; Esteve, M.; Panés, J.; Gisbert, J.P.; et al. Inflammatory bowel disease integral care units: Evaluation of a nationwide quality certification programme. The GETECCU experience. United Eur. Gastroenterol. J. 2021, 9, 766–772. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística (INE). Población por Provincias y Sexo. 2021. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=2852#!tabs-tabla (accessed on 2 September 2021).
- Burke, K.E.; Kochar, B.; Allegretti, J.R.; Winter, R.W.; Lochhead, P.; Khalili, H.; Colizzo, F.P.; Hamilton, M.J.; Chan, W.W.; Ananthakrishnan, A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 155–161. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Xie, D.; Lewis, J.; Trivedi, C.; Yang, Y.-X. Impact of Anti-Tumor Necrosis Factor and Thiopurine Medications on the Development of COVID-19 in Patients with Inflammatory Bowel Disease: A Nationwide Veterans Administration Cohort Study. Gastroenterology 2020, 159, 1545–1546.e1. [Google Scholar] [CrossRef]
- Chaparro, M.; Garre, A.; Núñez Ortiz, A.; Diz-Lois Palomares, M.T.; Rodríguez, C.; Riestra, S.; Vela, M.; Benítez, J.M.; Fernández Salgado, E.; Sánchez Rodríguez, E.; et al. Incidence, Clinical Characteristics and Management of Inflammatory Bowel Disease in Spain: Large-Scale Epidemiological Study. J. Clin. Med. 2021, 10, 2885. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mahmud, N.; Trivedi, C.; Reinisch, W.; Lewis, J.D. Risk factors for SARS-CoV-2 infection and course of COVID-19 disease in patients with IBD in the Veterans Affair Healthcare System. Gut 2021, 70, 1657–1664. [Google Scholar] [CrossRef]
- Ardizzone, S.; Ferretti, F.; Monico, M.C.; Carvalhas Gabrielli, A.M.; Carmagnola, S.; Bezzio, C.; Saibeni, S.; Bosani, M.; Caprioli, F.; Mazza, S.; et al. Lower incidence of COVID-19 in patients with inflammatory bowel disease treated with non-gut selective biologic therapy. J. Gastroenterol. Hepatol. 2021, 36, 3050–3055. [Google Scholar] [CrossRef]
- Derikx, L.A.A.P.; Lantinga, M.A.; de Jong, D.J.; van Dop, W.A.; Creemers, R.H.; Römkens, T.E.H.; Jansen, J.M.; Mahmmod, N.; West, R.L.; Tan, A.C.I.T.L.; et al. Clinical Outcomes of Covid-19 in Patients with Inflammatory Bowel Disease: A Nationwide Cohort Study. J. Crohn’s Colitis 2020, 15, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khan, A.; Chowdhry, M.; Bilal, M.; Kochhar, G.S.; Clarke, K. Risk of Severe Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 1575–1578.e4. [Google Scholar] [CrossRef] [PubMed]
- Wetwittayakhlang, P.; Albader, F.; Golovics, P.A.; Bessissow, T.; Bitton, A.; Afif, W.; Wild, G.; Lakatos, P.L. Clinical Outcomes of COVID-19 and Impact on Disease Course in Patients with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2021, 2021, 7591141. [Google Scholar] [CrossRef]
- Attauabi, M.; Poulsen, A.; Theede, K.; Pedersen, N.; Larsen, L.; Jess, T.; Rosager Hansen, M.; Verner-Andersen, M.K.; Haderslev, K.V.; Berg Lødrup, A.; et al. Prevalence and Outcomes of COVID-19 Among Patients with Inflammatory Bowel Disease—A Danish Prospective Population-Based Cohort Study. J. Crohn’s Colitis 2020, 15, 540–550. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Axelrad, J.; Halfvarson, J.; Khalili, H.; Larsson, E.; Lochhead, P.; Roelstraete, B.; Simon, T.G.; Söderling, J.; Olén, O. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. United Eur. Gastroenterol. J. 2021, 9, 177–192. [Google Scholar] [CrossRef]
- Guzzetta, G.; Riccardo, F.; Marziano, V.; Poletti, P.; Trentini, F.; Bella, A.; Andrianou, X.; Del Manso, M.; Fabiani, M.; Bellino, S.; et al. Impact of a Nationwide Lockdown on SARS-CoV-2 Transmissibility, Italy. Emerg. Infect. Dis. 2021, 27, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X.; et al. Association of Public Health Interventions with the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020, 323, 1915–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodyga, M.; Maciejewska, K.; Eder, P.; Waszak, K.; Stawczyk-Eder, K.; Dobrowolska, A.; Kaczka, A.; Gąsiorowska, A.; Stępień-Wrochna, B.; Cicha, M.; et al. Social distancing during COVID-19 pandemic among inflammatory bowel disease patients. J. Clin. Med. 2021, 10, 3689. [Google Scholar] [CrossRef] [PubMed]
- Allocca, M.; Chaparro, M.; Gonzalez, H.A.; Bosca-Watts, M.M.; Palmela, C.; D’Amico, F.; Zacharopoulou, E.; Kopylov, U.; Ellul, P.; Bamias, G.; et al. Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID-19: A Large Multinational Cohort Study. J. Clin. Med. 2020, 9, 3533. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Rahier, J.F.; Abreu, C.; MacMahon, E.; Hart, A.; van der Woude, C.J.; Gordon, H.; Adamina, M.; Viget, N.; Vavricka, S.; et al. Inflammatory bowel disease management during the COVID-19 outbreak:The ten do’s and don’ts from the ECCO-COVID task force. J. Crohn’s Colitis 2020, 14, S798–S806. [Google Scholar] [CrossRef]
- Bezzio, C.; Pellegrini, L.; Manes, G.; Arena, I.; Picascia, D.; Della Corte, C.; Devani, M.; Schettino, M.; Saibeni, S. Biologic therapies may reduce the risk of COVID-19 in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2020, 26, E107–E109. [Google Scholar] [CrossRef]
- Iborra, I.; Puig, M.; Marín, L.; Calafat, M.; Cañete, F.; Quiñones, C.; González-González, L.; Cardona, G.; Mañosa, M.; Domènech, E. Treatment Adherence and Clinical Outcomes of Patients with Inflammatory Bowel Disease on Biological Agents during the SARS-CoV-2 Pandemic. Dig. Dis. Sci. 2021, 66, 4191–4196. [Google Scholar] [CrossRef]
- The Lancet. COVID-19: Protecting health-care workers. Lancet 2020, 395, 922. [Google Scholar] [CrossRef]
- Bezzio, C.; Saibeni, S.; Variola, A.; Allocca, M.; Massari, A.; Gerardi, V.; Casini, V.; Ricci, C.; Zingone, F.; Amato, A.; et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 2020, 69, 1213–1217. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Nowak, J.K.; Lindstrøm, J.C.; Kalla, R.; Ricanek, P.; Halfvarson, J.; Satsangi, J. Age, Inflammation, and Disease Location Are Critical Determinants of Intestinal Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1151–1154.e2. [Google Scholar] [CrossRef]
- Lukin, D.J.; Kumar, A.; Hajifathalian, K.; Sharaiha, R.Z.; Scherl, E.J.; Longman, R.S. Baseline Disease Activity and Steroid Therapy Stratify Risk of COVID-19 in Patients with Inflammatory Bowel Disease. Gastroenterology 2020, 159, 1541–1544.e2. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Axelrad, J.E.; Malter, L.; Hong, S.; Chang, S.; Bosworth, B.; Hudesman, D. From the American Epicenter: Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the New York City Metropolitan Area. Inflamm. Bowel Dis. 2020, 27, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Bataille, P.; Amiot, A.; Claudepierre, P.; Paris, N.; Neuraz, A.; Lerner, I.; Garcelon, N.; Rance, B.; Grisel, O.; Moreau, T.; et al. Letter: Severe COVID-19 infection and biologic therapies—A cohort study of 7 808 patients in France. Aliment. Pharmacol. Ther. 2020, 52, 1245–1248. [Google Scholar] [PubMed]
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020, 395, 683–684. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, Y.; Tian, D.; Wang, C.; Wang, S.; Cheng, J.; Hu, M.; Fang, M.; Gao, Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal. Transduct. Target. Ther. 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, M.; Brenner, E.J.; Zhang, X.; Colombel, J.-F.; Kappelman, M.D.; Ungaro, R.C.; Gearry, R.B.; Kalpan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; et al. Physician Practice Patterns in Holding Inflammatory Bowel Disease Medications due to COVID-19, in the SECURE-IBD Registry. J. Crohns. Colitis 2021, 15, 860–863. [Google Scholar] [CrossRef]
- Attauabi, M.; Dahlerup, J.F.; Poulsen, A.; Hansen, M.R.; Verner-Andersen, M.K.; Eraslan, S.; Prahm, A.P.; Pedersen, N.; Larsen, L.; Jess, T.; et al. Outcomes and long-term effects of COVID-19 in patients with inflammatory bowel diseases—A Danish prospective population-based cohort study with individual-level data. J. Crohn’s Colitis 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Mertz, B.; Dijkstra, F.; Nielsen, J.; Kjeldsen, J. Post COVID-19 hospitalizations in patients with chronic inflammatory diseases—A nationwide cohort study. J. Autoimmun. 2021, 125, 102739. [Google Scholar]


| Clinical Characteristics | Cases n = 482 |
|---|---|
| Gender | |
| Male | 251 (52) |
| Female | 231 (48) |
| Age at COVID-19 diagnosis | 52 years (IQR 42–61) |
| IBD duration at COVID-19 diagnosis | 12 years (IQR 6–19) |
| Type of IBD, n (%) | |
| Crohn’s disease | 247 (51) |
| Ulcerative colitis | 221 (46) |
| Unclassified colitis | 14 (2.9) |
| Ulcerative colitis extent (%) | |
| E1 | 43 (19) |
| E2 | 80 (36) |
| E3 | 98 (44) |
| Crohn’s disease location, n (%) | |
| L1 | 114 (46) |
| L2 | 43 (17) |
| L3 | 88 (36) |
| L4 (isolated) | 3 (1.2) |
| Crohn’s disease behaviour, n (%) | |
| B1 | 144 (58) |
| B2 | 71 (29) |
| B3 | 47 (19) |
| Perianal | 59 (24) |
| B1 + perianal | 29 (12) |
| B2 + perianal | 18 (7.3) |
| B3 + perianal | 20 (8.1) |
| Extraintestinal manifestation, n (%) | 125 (26) |
| Family history of IBD, n (%) | 64 (13) |
| Smoking behaviour, n (%) | |
| Active | 53 (11) |
| Former smoker | 137 (28) |
| Never smoker | 268 (56) |
| IBD (Total) n = 482 | Crohn’s Disease n = 247 | Ulcerative Colitis n = 221 | p-Value * | |
|---|---|---|---|---|
| IBD Activity at COVID-19 Diagnosis | ||||
| Clinical remission | 385 (80) | 200 (81) | 173 (78) | 0.35 |
| Active disease | 97 (20) | 47 (19) | 48 (22) | 0.35 |
| Mild | 53 (11) | 26 (10.5) | 26 (12) | |
| Moderate | 42 (8.7) | 21 (8.5) | 20 (9) | |
| Severe | 2 (0.4) | 0 | 2 (0.9) | |
| IBD treatment | ||||
| None, n (%) | 62 (13) | 37 (15) | 23 (10.4) | 0.15 |
| 5-aminosalicylates, n (%) | 202 (42) | 49 (20) | 143 (65) | <0.0001 |
| Oral (oral and topic) | 197 (41) | 49 (20) | 138 (62) | |
| Topical (exclusive) | 5 (1) | 0 | 5 (2.3) | |
| Monotherapy | 131 (27) | 31 (12) | 91 (41) | |
| Systemic steroids 3 months before COVID-19 (oral or intravenous), n (%) | 53 (11) | 30 (12) | 21 (9.5) | 0.36 |
| Systemic steroids, n (%) | 26 (5.4) | 16 (6.4) | 8 (3.6) | 0.37 |
| Immunosuppressants (in monotherapy), n (%) | 113 (23) | 65 (26) | 56 (25) | 0.03 |
| Azathioprine | 90 (19) | 54 (22) | 46 (21) | 0.04 |
| Mercaptopurine | 8 (1.7) | 4 (1.6) | 2 (0.9) | 0.16 |
| Cyclosporine | 1 (0.2) | 0 | 1 (0.4) | 0.96 |
| Methotrexate | 9 (1.9) | 6 (2.4) | 3 (1.3) | 0.06 |
| Tacrolimus | 1 (0.2) | 1 (0.4) | 0 | 1 |
| Tofacitinib | 4 (0.8) | 0 | 4 (1.8) | 0.10 |
| Biologics (in monotherapy), n (%) | 117 (22) | 72 (29) | 35 (16) | 0.04 |
| Anti-TNF | 71 (15) | 42 (17) | 19 (8.6) | <0.0001 |
| Vedolizumab | 25 (5.2) | 12 (4.8) | 13 (5.9) | 0.75 |
| Ustekinumab | 21 (4.3) | 18 (7.3) | 3 (1.3) | 0.001 |
| Combotherapy, n (%) | 59 (12) | 45 (18) | 14 (6.3) | 0.02 |
| Anti-TNF plus thiopurines | 37 (7.7) | 28 (11) | 9 (4.1) | 0.02 |
| Anti-TNF plus methotrexate | 9 (1.9) | 6 (2.4) | 3 (1.3) | 0.62 |
| Vedolizumab plus thiopurines | 5 (1) | 4 (1.6) | 1 (0.4) | 0.73 |
| Vedolizumab plus methotrexate | 1 (0.2) | 0 | 1 (0.4) | 0.78 |
| Ustekinumab plus thiopurines | 5 (1) | 5 (2) | 0 | 0.55 |
| Ustekinumab plus methotrexate | 2 (0.4) | 2 (0.8) | 0 | 0.98 |
| Route of Contagion, n (%) | |
| Unknown | 242 (50) |
| Intrafamilial transmission | 108 (22) |
| Occupational | 96 (20) |
| Travel | 8 (1.7) |
| Occupational Risk, n (%) | 133 (28) |
| Healthcare | 85 (18) |
| Basic services (supermarket cashiers, market clerks, pharmacy) | 18 (3.7) |
| Education | 15 (3) |
| Police and fireperson | 5 (1) |
| Closed institutions | 2 (0.4) |
| Veterinary, animal control worker or conservation and forest technician | 4 (0.8) |
| Risk Variable | Patients with Total Lockdown (n = 229) | Patients without Total Lockdown (n = 225) | p-Value |
|---|---|---|---|
| Route of contagion, n (%) | |||
| Intrafamilial transmission | 70 (31) | 38 (17) | 0.001 |
| Infection on March 2020 | 47 (20) | 26 (12) | 0.007 |
| Infection on April–July 2020 | 23 (10) | 12 (5.3) | 0.034 |
| Occupational | 19 (8.3) | 77 (34) | <0.0001 |
| Infection on March 2020 | 19 (8.2) | 48 (21) | <0.0001 |
| Infection on April–July 2020 | 0 | 29 (13) | <0.0001 |
| Travel | 3 (1.3) | 5 (2.2) | 0.69 |
| Infection on March 2020 | 3 (1.3) | 5 (2.2) | 0.69 |
| Infection on April–July 2020 | - | - | |
| Unknown | 137 (60) | 105 (47) | 0.005 |
| Infection on March 2020 | 83 (36) | 69 (31) | 0.167 |
| Infection on April–July 2020 | 54 (24) | 36 (16) | 0.003 |
| Occupational risk, n (%) | |||
| Occupational risk (all) | 38 (17) | 92 (41) | <0.0001 |
| Healthcare | 18 (7.9) | 65 (29) | <0.0001 |
| ||||||||||
| Outcome | Hospitalised | ICU Admission | Severe COVID-19 | Death | ||||||
| COVID-19-EII n = 482 | SECURE-IBD n = 6438 | COVID-19-EII n = 482 | SECURE-IBD n = 6438 | COVID-19-EII n = 482 | SECURE-IBD n = 6438 | COVID-19-EII n = 482 | SECURE-IBD n = 6438 | |||
| n = 168 (35%) | n = 977 (15%) | n = 13 (2.6%) | n = 184 (2.8%) | n = 38 * (7.8%) | n = 257 ** (3.9%) | n = 18 (3.7%) | n = 104 (1.6%) | |||
| ||||||||||
| DRUG | Total | OUTCOMES | ||||||||
| Hospitalised | ICU | Severe COVID-19 | Death | |||||||
| Cohort | COVID-19-EII n = 482 | SECURE-IBD n = 6438 | COVID-19-EII n = 168 | SECURE-IBD n = 977 | COVID-19-EII n = 13 | SECURE-IBD n = 184 | COVID-19-EII * n = 38 | SECURE-IBD ** n = 257 | COVID-19-EII n = 18 | SECURE-IBD n = 104 |
| 5-aminosalicylates, | 202 (42) | 1924 (30) | 79 (47) | 411 (42) | 10 (77) | 81 (44) | 24 (63) | 118 (46) | 9 (50) | 53 (51) |
| Alone | 131 (27) | - | 52 (31) | - | 5 (38) | - | 16 (42) | - | 6 (33) | - |
| With other IBD drugs | 71 (15) | - | 27 (16) | - | 5 (28) | - | 8 (21) | - | 3 (17) | - |
| Systemic steroids | 26 (5.4) | 414 (6.4) | 11 (6.5) | 146 (15) | 1 (7.7) | 41 (22) | 4 (10.5) | 53 (21) | 1 (5.6) | 28 (27) |
| Thiopurines (monotherapy) | 108 (22) | 551 (8.6) | 38 (23) | 114 (12) | 3 (23) | 26 (14) | 5 (13) | 33 (13) | 1 (5.6) | 11 (10.6) |
| Methotrexate (monotherapy) | 9 (1.9) | 49 (0.8) | 4 (2.4) | 13 (1.3) | 1 (7.7) | 1 (0.5) | 2 (5.3) | 3 (1.2) | 1 (5.6) | 2 (1.9) |
| Anti-TNF (monotherapy) | 71 (15) | 2082 (32) | 11 (6.5) | 178 (18) | 2 (15) | 24 (13) | 3 (7.9) | 31 (12) | 2 (11) | 10 (9.6) |
| Anti-TNF in combotherapy | 46 (9.5) | 636 (9.9) | 20 (12) | 91 (9.3) | 0 | 17 (9) | 2 (5.3) | 21 (8.2) | 2 (11) | 6 (5.8) |
| Vedolizumab | 30 (6.2) | 706 (11) | 15 (8.9) | 94 (9.6) | 0 | 21 (11) | 4 (10.5) | 28 (10.9) | 2 (11) | 9 (8.6) |
| Ustekinumab | 28 (5.8) | 602 (9) | 6 (3.6) | 50 (5.1) | 1 (7.7) | 9 (4.9) | 1 (2.6) | 11 (4.3) | 1 (5.6) | 5 (4.8) |
| Tofacitinib | 4 (0.8) | 103 (1.6) | 2 (1.2) | 12 (1.2) | 0 | 4 (2.2) | 0 | 4 (1.5) | 0 | 1 (0.9) |
| 3 Months Follow-Up (n = 462) | 12 Months Follow-Up (n = 451) | |
|---|---|---|
| COVID-19 sequelae, n (%) | 65 (14) | 72 (15) |
| Psychologic sequelae, n (%) | 20 (4.3) | 15 (3.3) |
| Physical sequelae, n (%) | 55 (12) | 67 (15) |
| Asthenia | 21 (4.5) | 22 (4.8) |
| Myalgia/Arthralgia | 7 (1.5) | 3 (0.7) |
| Anosmia | 4 (0.9) | 7 (1.5) |
| Dyspnoea | 4 (0.9) | 6 (1.3) |
| Odynophagia | 2 (0.4) | 2 (0.4) |
| Dysgeusia | 2 (0.4) | 2 (0.4) |
| Hair loss | 1 (0.2) | 1 (0.2) |
| Pulmonary fibrosis | 1 (0.2) | 3 (0.6) |
| Bronchial hyperreactivity | 1 (0.2) | 1 (0.2) |
| Deep venous thrombosis/pulmonary thrombosis | 1 (0.2) | 1 (0.2) |
| Headache | 1 (0.2) | 6 (1.3) |
| Obstructive pulmonary disease | 1 (0.2) | 3 (0.6) |
| Paraesthesia | 1 (0.2) | 3 (0.6) |
| Wegener’s vasculitis | - | 1 (0.2) |
| Immunosuppression withdrawal, n (%) | 65 (14) | 6 (1.3) |
| Transient | 58 (13) | 1 (0.2) |
| Definitive | 7 (1.5) | 5 (1.1) |
| De-escalation from combo to monotherapy | 13 (2.9) | 1 (0.2) |
| Patients requiring Immunosuppression initiation or modification, n (%) | 12 (2.6) | 43 * (9.5) |
| Systemic corticosteroids | 1 (0.2) | 7 (1.6) |
| Thiopurines | 2 (0.4) | 5 (1.1) |
| Methotrexate | 0 | 1 (0.2) |
| Anti-TNF | 5 (1) | 18 (4) |
| Vedolizumab | 1 (0.2) | 7 (1.6) |
| Ustekinumab | 2 (0.4) | 12 (2.7) |
| Tofacitinib | 1 (0.2) | 6 (1.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabana, Y.; Marín-Jiménez, I.; Rodríguez-Lago, I.; Vera, I.; Martín-Arranz, M.D.; Guerra, I.; Gisbert, J.P.; Mesonero, F.; Benítez, O.; Taxonera, C.; et al. Nationwide COVID-19-EII Study: Incidence, Environmental Risk Factors and Long-Term Follow-Up of Patients with Inflammatory Bowel Disease and COVID-19 of the ENEIDA Registry. J. Clin. Med. 2022, 11, 421. https://doi.org/10.3390/jcm11020421
Zabana Y, Marín-Jiménez I, Rodríguez-Lago I, Vera I, Martín-Arranz MD, Guerra I, Gisbert JP, Mesonero F, Benítez O, Taxonera C, et al. Nationwide COVID-19-EII Study: Incidence, Environmental Risk Factors and Long-Term Follow-Up of Patients with Inflammatory Bowel Disease and COVID-19 of the ENEIDA Registry. Journal of Clinical Medicine. 2022; 11(2):421. https://doi.org/10.3390/jcm11020421
Chicago/Turabian StyleZabana, Yamile, Ignacio Marín-Jiménez, Iago Rodríguez-Lago, Isabel Vera, María Dolores Martín-Arranz, Iván Guerra, Javier P. Gisbert, Francisco Mesonero, Olga Benítez, Carlos Taxonera, and et al. 2022. "Nationwide COVID-19-EII Study: Incidence, Environmental Risk Factors and Long-Term Follow-Up of Patients with Inflammatory Bowel Disease and COVID-19 of the ENEIDA Registry" Journal of Clinical Medicine 11, no. 2: 421. https://doi.org/10.3390/jcm11020421