The Thromboembolic Predictability of CHA2DS2-VASc Scores Using Different Echocardiographic Criteria for Congestive Heart Failure in Korean Patients with Nonvalvular Atrial Fibrillation
Abstract
:1. Introduction
2. Methods
2.1. Study Sample
2.2. Echocardiography
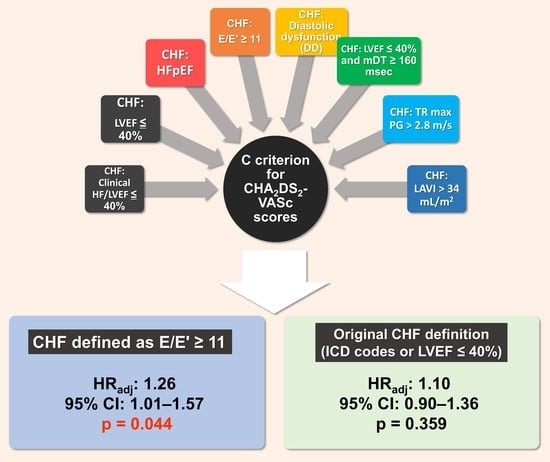
2.3. Definitions of Various C Criteria
2.4. Medication and Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
3.3. Baseline Echocardiography Findings
3.4. Multivariate Analysis Using Different Definitions for CHF
3.5. Survival Analysis
3.6. C-Statistics Analysis
3.7. Discussion/Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzone, C.; Cioffi, G.; Di Nora, C.; Barbati, G.; Guidetti, F.; Faggiano, P.; Gaibazzi, N.; Faganello, G.; Borca, E.C.; Di Lenarda, A. Prognostic role of cardiac calcifications in primary prevention: A powerful marker of adverse outcome highly dependent on underlying cardiac rhythm. Int. J. Cardiol. 2018, 258, 262–268. [Google Scholar] [CrossRef]
- Ikeda, T. Which Score Should Be Used for Risk Stratification of Ischemic Stroke in Patients With Atrial Fibrillation. Circ. J. 2014, 78, 1331–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Marin, F.; Camm, A.J.; Lip, G.Y.H. Stroke and Thromboembolism in Atrial Fibrillation. Circ. J. 2012, 76, 2289–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friberg, L.; Lund, L.H. Heart failure: A weak link in CHA2 DS2 -VASc. ESC Heart Fail 2018, 5, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jameson, J.L. Harrison’s Principles of Internal Medicine, 20th ed.; McGraw-Hill Education: New York, NY, USA, 2018; p. 2, Volumes (xli, 3528, I-3214 pages). [Google Scholar]
- Konstam, M.A.; Abboud, F.M. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann. Intern. Med. 1992, 116, 1–5. [CrossRef] [PubMed]
- Stroke Prevention in Atrial Fibrillation, I. Risk factors for thromboembolism during aspirin therapy in patients with atrial fibrillation: The stroke prevention in atrial fibrillation study. J. Stroke Cerebrovasc. Dis. 1995, 5, 147–157. [Google Scholar] [CrossRef]
- Aronow, W.S.; Ahn, C.; Kronzon, I.; Gutstein, H. Risk factors for new thromboembolic stroke in patients > or = 62 years of age with chronic atrial fibrillation. Am. J. Cardiol. 1998, 82, 119–121. [Google Scholar] [CrossRef]
- Stollberger, C.; Chnupa, P.; Kronik, G.; Brainin, M.; Finsterer, J.; Schneider, B.; Slany, J. Transesophageal echocardiography to assess embolic risk in patients with atrial fibrillation. Ann. Intern. Med. 1998, 128, 630–638. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; McBride, R.; Rothbart, R.M.; Asinger, R.W. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: Analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 1999, 30, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Pearce, L.A.; Rothbart, R.M.; McAnulty, J.H.; Asinger, R.W.; Halperin, J.L. Stroke with intermittent atrial fibrillation: Incidence and predictors during aspirin therapy. Stroke Prevention in Atrial Fibrillation Investigators. J. Am. Coll. Cardiol. 2000, 35, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Stollberger, C.; Chnupa, P.; Abzieher, C.; Langer, T.; Finsterer, J.; Klem, I.; Hartl, E.; Wehinger, C.; Schneider, B. Mortality and rate of stroke or embolism in atrial fibrillation during long-term follow-up in the embolism in left atrial thrombi (ELAT) study. Clin. Cardiol. 2004, 27, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.; Ezekowitz, J.A.; Lewis, B.S.; Gersh, B.J.; van Diepen, S.; Amerena, J.; Bartunek, J.; Commerford, P.; Oh, B.H.; Harjola, V.P.; et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Circ. Heart Fail. 2013, 6, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, R.K.; Hohnloser, S.H.; Pfeffer, M.A.; Yuan, F.; Hart, R.G.; Yusuf, S.; Connolly, S.J.; McAlister, F.A.; Healey, J.S. Relationship between degree of left ventricular dysfunction, symptom status, and risk of embolic events in patients with atrial fibrillation and heart failure. Stroke 2015, 46, 667–672. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.W.; Lee, B.K.; Kim, H.J.; Pyun, W.B.; Byun, K.H.; Rim, S.J.; Chung, N. Assessment of left atrial appendage filling pattern by using intravenous administration of microbubbles: Comparison between mitral stenosis and mitral regurgitation. J. Am. Soc. Echocardiogr. 2001, 14, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.E.; Pearce, L.A.; Hart, R.G.; Zabalgoitia, M.; Asinger, R.W.; Safford, R.; Halperin, J.L. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 1999, 12, 1080–1087. [Google Scholar] [CrossRef]
- Jang, A.Y.; Yu, J.; Park, Y.M.; Shin, M.S.; Chung, W.J.; Moon, J. Cardiac Structural or Functional Changes Associated with CHA2DS2-VASc Scores in Nonvalvular Atrial Fibrillation: A Cross-Sectional Study Using Echocardiography. J. Cardiovasc. Imaging 2018, 26, 135–143. [Google Scholar] [CrossRef]
- Sohn, D.W.; Song, J.M.; Zo, J.H.; Chai, I.H.; Kim, H.S.; Chun, H.G.; Kim, H.C. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J. Am. Soc. Echocardiogr. 1999, 12, 927–931. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Kopelen, H.A.; Quinones, M.A. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation 1996, 94, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.P.; Rhode, E.A.; Peoples, S.A.; Kines, H. Left ventricular function in mammals of greatly different size. Circ. Res. 1962, 10, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Shim, C.Y.; Park, J.H.; Nam, C.M.; Uhm, J.S.; Joung, B.; Lee, M.H.; Pak, H.N. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J. Cardiol. 2016, 68, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Shim, C.Y.; Wi, J.; Joung, B.; Ha, J.W.; Lee, M.H.; Pak, H.N. Left ventricular diastolic function is closely associated with mechanical function of the left atrium in patients with paroxysmal atrial fibrillation. Circ. J. 2013, 77, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotecha, D.; Mohamed, M.; Shantsila, E.; Popescu, B.A.; Steeds, R.P. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace 2017, 19, 1427–1438. [Google Scholar] [CrossRef]
- Matsukida, K.; Kisanuki, A.; Toyonaga, K.; Murayama, T.; Nakashima, H.; Kumanohoso, T.; Yoshifuku, S.; Saigo, M.; Abe, S.; Hamasaki, S.; et al. Comparison of transthoracic Doppler echocardiography and natriuretic peptides in predicting mean pulmonary capillary wedge pressure in patients with chronic atrial fibrillation. J. Am. Soc. Echocardiogr. 2001, 14, 1080–1087. [Google Scholar] [CrossRef]
- Rossi, A.; Cicoira, M.; Zanolla, L.; Sandrini, R.; Golia, G.; Zardini, P.; Enriquez-Sarano, M. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 1425. [Google Scholar] [CrossRef] [Green Version]
- Faganello, G.; Doimo, S. Cardiac imaging in patients with acute or chronic heart failure. Minerva Cardioangiol. 2017, 65, 589–600. [Google Scholar] [CrossRef]
- Gupta, D.K.; Giugliano, R.P.; Ruff, C.T.; Claggett, B.; Murphy, S.; Antman, E.; Mercuri, M.F.; Braunwald, E.; Solomon, S.D. The Prognostic Significance of Cardiac Structure and Function in Atrial Fibrillation: The ENGAGE AF-TIMI 48 Echocardiographic Substudy. J. Am. Soc. Echocardiogr. 2016, 29, 537–544. [Google Scholar] [CrossRef] [Green Version]


| All (n = 4200) | CATE(−) (n = 4029) | CATE(+) (n = 171) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years) | 71 ± 12 | 71 ± 12 | 73 ± 11 | 0.005 |
| Men, n (%) | 2487 (59) | 2391 (59) | 95 (56) | 0.323 |
| Congestive heart failure | ||||
| ICD code or LVEF ≤ 40%, n (%) | 1962 (47) | 1885 (47) | 77 (45) | 0.652 |
| LVEF ≤ 40%, n (%) | 895 (21) | 858 (21) | 37 (22) | 0.924 |
| HFpEF, n (%) | 495 (12) | 476 (12) | 19 (11) | 0.904 |
| E/E’ ≥ 11, n (%) | 2929 (71) | 3800 (71) | 129 (76) | 0.168 |
| LAVI>34 mL/m2, n (%) | 3163 (77) | 3029 (77) | 134 (80) | 0.197 |
| TR max PG>2.8 m/s, n (%) | 1105 (26) | 1051 (26) | 54 (32) | 0.070 |
| DD, n (%) | 1233 (29) | 1183 (29) | 50 (29) | 1.000 |
| LVEF ≤ 40% and mDT ≤ 160 ms, n (%) | 614 (15) | 593 (15) | 21 (13) | 0.573 |
| ICD codes for CHF, n (%) | 1453 (35) | 1400 (35) | 53 (31) | 0.326 |
| Hypertension, n (%) | 1641 (39) | 1574 (39) | 67 (39) | 1.000 |
| Diabetes mellitus, n (%) | 751 (18) | 723 (18) | 28 (16) | 0.684 |
| Secondary prevention for TE, n(%) | 908 (22) | 876 (22) | 32 (19) | 0.200 |
| Ischemic stroke, n (%) | 792 (19) | 769 (19) | 23 (14) | 0.072 |
| TIA, n (%) | 96 (2) | 92 (2) | 4 (2) | 0.797 |
| Systemic/pulmonary TE, n (%) | 110 (3) | 102 (3) | 8 (5) | 0.088 |
| Peripheral arterial disease, n (%) | 78 (2) | 75 (2) | 2 (1) | 0.497 |
| Myocardial infarction, n (%) | 199 (5) | 192 (5) | 7 (4) | 0.854 |
| Aortic plaque, n (%) | 110 (3) | 102 (3) | 8 (5) | 0.088 |
| Systolic blood pressure (mm Hg) | 125 ± 27 | 125 ± 27 | 123 ± 24 | 0.513 |
| Diastolic blood pressure (mm Hg) | 74 ± 14 | 74 ± 14 | 72 ± 15 | 0.176 |
| Heart rate (bpm) | 89 ± 26 | 89 ± 26 | 86 ± 21 | 0.303 |
| Height (cm) | 162 ± 11 | 162 ± 11 | 160 ± 12 | 0.019 |
| Weight (kg) | 64 ± 11 | 64 ± 13 | 61 ± 16 | 0.003 |
| BMI | 24.4 ± 9.5 | 24.4 ± 7.6 | 25.3 ± 29.4 | 0.195 |
| Mean CHA2DS2-VASc score | 3.2 ± 1.9 | 3.2 ± 1.9 | 3.4 ± 1.7 | 0.227 |
| Medication or Procedures | ||||
| RFCA/CV | 120 (3) | 119 (3) | 1 (1) | 0.094 |
| Anticoagulation, n (%) | 1449 (34) | 1421 (35) | 29 (16) | <0.001 |
| VKA, n (%) | 845 (20) | 834 (21) | 11 (6) | |
| NOAC, n (%) | 604 (14) | 587 (15) | 17 (10) | |
| Duration (months) | 4.4 ± 8.2 | 4.5 ± 8.3 | 2.1 ± 6.2 | <0.001 |
| Antiplatelet, n (%) | 766 (18) | 188 (21) | 578 (17) | 0.008 |
| Duration (months) | 2.1 ± 6.2 | 2.2 ± 6.3 | 0.8 ± 4.2 | <0.001 |
| Antiarrhythmics, n (%) | 400 (10) | 398 (10) | 2 (1) | <0.001 |
| Beta blockers, n (%) | 1129 (27) | 1104 (27) | 25 (15) | <0.001 |
| Calcium channel blockers, n (%) | 289 (7) | 283 (7) | 6 (4) | 0.088 |
| ACEi/ARBs, n (%) | 1385 (33) | 1360 (34) | 25 (15) | <0.001 |
| Diuretics, n (%) | 1288 (31) | 1260 (31) | 28 (16) | <0.001 |
| 2-year clinical events | ||||
| Median follow up (months) | 10.6 (2.0–24.0) | 11.3 (2.3–24.0) | 1.5 (0–8.9) | <0.001 |
| CATE, n (%) | 171 (4.1) | 0 (0) | 171 (4.1) | <0.001 |
| Ischemic stroke, n (%) | 82 (2.0) | 0 (0) | 82 (48) | <0.001 |
| TIA, n (%) | 20 (0.5) | 0 (0) | 20 (11.7) | <0.001 |
| NCT, n (%) | 77 (1.8) | 0 (0) | 77 (45.0) | <0.001 |
| All (n = 4200) | CATE(−) (n = 4029) | CATE(+) (n = 171) | p | |
|---|---|---|---|---|
| LVEF (%) | 52 ± 16 | 52 ± 16 | 53 ± 15 | 0.952 |
| LVEDD (mm) | 50 ± 6 | 50 ± 6 | 50 ± 7 | 0.532 |
| LVESD (mm) | 35 ± 10 | 35 ± 10 | 35 ± 8 | 0.642 |
| LVEDV (mL) | 77 ± 37 | 77 ± 37 | 72 ± 35 | 0.182 |
| LVESV (mL) | 42 ± 31 | 43 ± 31 | 41 ± 30 | 0.523 |
| BSA (m2) | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.3 | <0.001 |
| LVEDD/BSA (mm/m2) | 30 ± 5 | 30 ± 5 | 31 ± 5 | 0.012 |
| LVESD/BSA (mm/m2) | 21 ± 6 | 21 ± 6 | 22 ± 5 | 0.194 |
| LVEDV/BSA (mL/m2) | 46 ± 21 | 46 ± 21 | 44 ± 19 | 0.552 |
| LVESV/BSA (mL/m2) | 25 ± 18 | 25 ± 18 | 25 ± 17 | 0.848 |
| LA diameter (mm) | 45 ± 16 | 45 ± 16 | 45 ± 16 | 0.857 |
| LVMI (g/m2) | 97 ± 31 | 97 ± 31 | 100 ± 29 | 0.251 |
| RWT | 0.37 ± 0.12 | 0.37 ± 0.12 | 0.37 ± 0.08 | 0.716 |
| LAVI (mL/m2) | 49 ± 22 | 49 ± 23 | 49 ± 20 | 0.985 |
| E/E’ | 13.4 ± 6.2 | 13.3 ± 6.1 | 13.8 ± 6.7 | 0.287 |
| Septal E’ velocity (cm/s) | 7 ± 3 | 7 ± 3 | 7 ± 2 | 0.217 |
| TR velocity max (m/s) | 2.5 ± 0.4 | 2.5 ± 0.4 | 2.6 ± 0.4 | 0.004 |
| mDT (ms) | 119 ± 114 | 119 ± 116 | 115 ± 70 | 0.728 |
| LVH, n (%) | 1370 (33) | 1308 (33) | 62 (36) | 0.318 |
| Definition of C Criterion of CHA2DS2-VASc Score | CATE | ||
|---|---|---|---|
| Adjusted HR | 95% CI | p | |
| ICD code or LVEF ≤ 40% | 1.10 | 0.90–1.36 | 0.359 |
| LVEF ≤ 40% | 1.19 | 0.97–1.47 | 0.100 |
| HFpEF | 1.07 | 0.88–1.31 | 0.493 |
| E/E’ ≥ 11 | 1.26 | 1.01–1.57 | 0.044 * |
| LAVI > 34 mL/m2 | 1.78 | 0.94–1.48 | 0.156 |
| TR max PG > 2.8 m/s | 1.14 | 0.93–1.40 | 0.211 |
| LVEF ≤ 40% and mDT ≥ 160 ms | 1.16 | 0.85–1.57 | 0.348 |
| DD | 1.14 | 0.93–1.40 | 0.204 |
| ICD code | 1.01 | 0.82–1.24 | 0.939 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | |
| CHA2DS2-VASc (E/E’ ≥ 11) | 1.26 (1.01–1.57) | 0.044 * | - | - | - | - |
| CHA2DS2-VASc (ICD code or LVEF ≤ 40%) | - | - | 1.10 (0.90–1.36) | 0.359 | - | - |
| CHA2DS2-VASc (LVEF ≤ 40%) | - | - | - | - | 1.19 (0.97–1.47) | 0.100 |
| RFCA/CV | 0.86 (0.11–6.37) | 0.856 | 0.81 (0.11–6.25) | 0.840 | 0.80 (0.10–6.19) | 0.833 |
| Anticoagulation | 0.54 (0.28–1.04) | 0.064 | 0.55 (0.29–1.06) | 0.074 | 0.54 (0.28–10.5) | 0.068 |
| Antiplatelets | 0.34 (0.18–0.62) | 0.001 * | 0.33 (0.18–0.62) | <0.001 * | 0.33 (0.18–0.62) | <0.001 * |
| Antiarrhythmics | 0.17 (0.04–0.71) | 0.016 * | 0.16 (0.04–0.68) | 0.157 | 0.16 (0.04–0.70) | 0.015 * |
| ACEi/ARBs | 0.41 (0.26–0.63) | <0.001 * | 0.42 (0.27–0.64) | <0.001 * | 0.41 (0.27–0.64) | <0.001 * |
| BBs | 0.77 (0.50–1.20) | 0.250 | 0.77 (0.49–1.20) | 0.241 | 0.77 (0.50–1.20) | 0.246 |
| CCBs | 0.51 (0.22–1.15) | 0.204 | 0.52 (0.23–1.17) | 0.115 | 0.52 (0.23–1.17) | 0.115 |
| Diuretics | 0.56 (0.36–0.85) | 0.006 * | 0.56 (0.37–0.85) | 0.006 * | 0.55 (0.36–0.84) | 0.006 * |
| Anticoagulation duration (months) | 0.98 (0.95–1.02) | 0.391 | 0.98 (0.94–1.02) | 0.359 | 0.98 (0.95–1.02) | 0.381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, A.Y.; Kang, W.C.; Park, Y.M.; Ha, K.; Seo, J.; Oh, P.C.; Lee, K.; Moon, J. The Thromboembolic Predictability of CHA2DS2-VASc Scores Using Different Echocardiographic Criteria for Congestive Heart Failure in Korean Patients with Nonvalvular Atrial Fibrillation. J. Clin. Med. 2022, 11, 300. https://doi.org/10.3390/jcm11020300
Jang AY, Kang WC, Park YM, Ha K, Seo J, Oh PC, Lee K, Moon J. The Thromboembolic Predictability of CHA2DS2-VASc Scores Using Different Echocardiographic Criteria for Congestive Heart Failure in Korean Patients with Nonvalvular Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(2):300. https://doi.org/10.3390/jcm11020300
Chicago/Turabian StyleJang, Albert Youngwoo, Woong Chol Kang, Yae Min Park, Kyungeun Ha, Jeongduk Seo, Pyung Chun Oh, Kyounghoon Lee, and Jeonggeun Moon. 2022. "The Thromboembolic Predictability of CHA2DS2-VASc Scores Using Different Echocardiographic Criteria for Congestive Heart Failure in Korean Patients with Nonvalvular Atrial Fibrillation" Journal of Clinical Medicine 11, no. 2: 300. https://doi.org/10.3390/jcm11020300