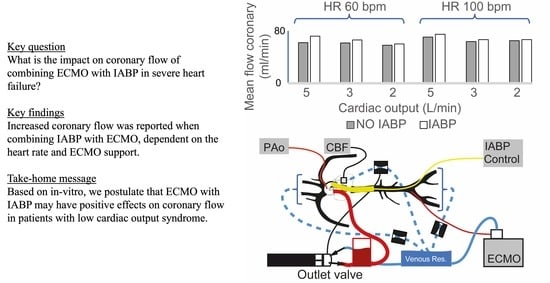
To Balloon or Not to Balloon? The Effects of an Intra-Aortic Balloon-Pump on Coronary Artery Flow during Extracorporeal Circulation Simulating Normal and Low Cardiac Output Syndromes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systemic Circulation Model
2.2. Coronary Artery Resistance Model
2.3. Extracorporeal (Membrane Oxygenation)
2.4. Intra-Aortic Balloon Counter-Pulsation
2.5. Pressure and Flow Measurements
2.6. Data Acquisition and Post-Processing
3. Results
4. Discussion
- (1)
- The performance of an IABP is dependent on heart rate in the absence or presence of ECMO [11]. The increase in coronary artery flow was more important in low heart rate conditions (HR 60 bpm). This would seem rational, as the time of balloon inflation is longer at a lower heart rate (time constant of duration) [14]. Indeed, the diastolic times in our model scenarios were about 650 and 390 ms for heart rates of 60 bpm and 100 bpm, respectively. Additionally, the dynamic effects during balloon inflation/deflation probably play a role at a higher HR, as coupling and resistance to retrograde flow from the femoral arteries (ECMO re-injection flow) may be increased.
- (2)
- The IABP has a positive impact on coronary artery blood flow perfusion in each of the experimental scenarios of LV heart failure, independent of the total cardiac output. We may be able to predict the role of IABP on coronary flow during VA-ECMO weaning by analyzing our results from an inverse timeline (Table 1 and Table 2).
- (3)
- The contribution of the IABP balloon to coronary artery perfusion at a constant flow rate (5 L/min) is less when the flow is primarily retrograde (supplied by the ECMO) than when the flow is entirely anterograde (absence of ECMO). One hypothesis is that the balloon may cause a resistance in the aorta that impedes the retrograde flow coming from the femoral artery ECMO cannula and that this occurs mainly during inflation and diastole (time of coronary artery perfusion). The present hypothesis could be investigated further using a model where the injecting cannula is placed in the right subclavian artery.
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werdan, K.; Gielen, S.; Ebelt, H.; Hochman, J.S. Mechanical circulatory support in cardiogenic shock. Eur. Heart J. 2014, 35, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldetti, L.; Pagnesi, M.; Gramegna, M.; Belletti, A.; Beneduce, A.; Pazzanese, V.; Calvo, F.; Sacchi, S.; Van Mieghem, N.M.; den Uil, C.A.; et al. Intra-Aortic Balloon Pumping in Acute Decompensated Heart Failure with Hypoperfusion: From Pathophysiology to Clinical Practice. Circ. Heart Fail. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hachamovitch, R.; Makkar, R.; Ramzy, D.; Moriguchi, J.D.; Arabia, F.A.; Esmailian, F.; Azarbal, B. Lack of Survival Benefit Found with Use of Intraaortic Balloon Pump in Extracorporeal Membrane Oxygenation: A Pooled Experience of 1517 Patients. J. Invasive Cardiol. 2015, 27, 453–458. [Google Scholar] [PubMed]
- Li, Y.N.; Yan, S.J.; Gao, S.Z.; Liu, M.Y.; Lou, S.; Liu, G.; Ji, B.Y.; Gao, B.R. Effect of an intra-aortic balloon pump with venoarterial extracorporeal membrane oxygenation on mortality of patients with cardiogenic shock: A systematic review and meta-analysis. Eur. J. Cardio-Thorac. 2019, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Liao, C.W.; Wang, C.H.; Chi, N.H.; Yu, H.Y.; Chou, N.K.; Hwang, J.J.; Lin, J.L.; Chiang, F.T.; Chen, Y.S. Effects of Additional Intra-aortic Balloon Counter-Pulsation Therapy to Cardiogenic Shock Patients Supported by Extra-corporeal Membranous Oxygenation. Sci. Rep. 2016, 6, 23838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhajosyula, S.; O’Horo, J.C.; Antharam, P.; Ananthaneni, S.; Vallabhajosyula, S.; Stulak, J.M.; Eleid, M.F.; Dunlay, S.M.; Gersh, B.J.; Rihal, C.S.; et al. Concomitant Intra-Aortic Balloon Pump Use in Cardiogenic Shock Requiring Veno-Arterial Extracorporeal Membrane Oxygenation. Circ. Cardiovasc. Interv. 2018, 11, e006930. [Google Scholar] [CrossRef] [PubMed]
- Kimman, J.R.; Van Mieghem, N.M.; Endeman, H.; Brugts, J.J.; Constantinescu, A.A.; Manintveld, O.C.; Dubois, E.A.; den Uil, C.A. Mechanical Support in Early Cardiogenic Shock: What Is the Role of Intra-aortic Balloon Counterpulsation? Curr. Heart Fail. Rep. 2020, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P.; Merenda, F.; Perren, F.; Rufenacht, D.; Stergiopulos, N. Validation of a one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H208–H222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, N.S. Formula for the viscosity of a glycerol-water mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Spaan, J. Coronary Blood Flow; Springer: Dordrecht, The Netherlands, 1991; p. 396. [Google Scholar]
- Imamura, T.; Juricek, C.; Nguyen, A.; Chung, B.; Rodgers, D.; Sayer, G.; Sarswat, N.; Kim, G.; Raikhelkar, J.; Ota, T.; et al. Predictors of Hemodynamic Improvement and Stabilization Following Intraaortic Balloon Pump Implantation in Patients with Advanced Heart Failure. J. Invasive Cardiol. 2018, 30, 56–61. [Google Scholar] [PubMed]
- Bendjelid, K. IABP and cardiogenic shock: A heartbreaking story. Am. Heart J. 2018, 199, 178–180. [Google Scholar] [CrossRef]
- Rigamonti, F.; Graf, G.; Merlani, P.; Bendjelid, K. The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: A retrospective cohort study. Crit. Care Med. 2013, 41, 2484–2491. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Terrovitis, J.; Kanakakis, J.; Stamatelopoulos, K.S.; Protogerou, A.D.; Lekakis, J.P.; Nanas, J.N.; Stamatelopoulos, S.F. Heart rate effect on hemodynamics during mechanical assistance by the intra-aortic balloon pump. Int. J. Artif. Organs 2002, 25, 1160–1165. [Google Scholar] [CrossRef] [PubMed]






| Pump Output (L/min) | 5 | 4 | 3 | 2 | ||||
|---|---|---|---|---|---|---|---|---|
| ECMO (L/min) | - | 1 | 2 | 3 | ||||
| IABP function | - | 100% | - | 100% | - | 100% | - | 100% |
| Mean coronary flow (mL/min) | 62 | 72 | 65.6 | 70.4 | 61.5 | 66.1 | 58 | 60 |
| Mean coronary flow increase (%) | 16 | 7.3 | 7.5 | 3.4 | ||||
| Mean pressure aortic arch (mmHg) | 93 | 101 | 92 | 99 | 87 | 94 | 85 | 89 |
| Mean ascending aorta flow (mL/min) | 4859 | 4832 | 3982 | 3984 | 3039 | 3078 | 2045 | 2036 |
| Pump Output (L/min) | 5 | 4 | 3 | 2 | ||||
|---|---|---|---|---|---|---|---|---|
| ECMO (L/min) | - | 1 | 2 | 3 | ||||
| IABP function | - | 100% | - | 100% | - | 100% | - | 100% |
| Mean coronary flow (mL/min) | 73 | 77.4 | 68 | 71.4 | 66 | 69 | 67.4 | 69.1 |
| Mean coronary flow increase (%) | 6 | 5 | 4.5 | 2.5 | ||||
| Mean pressure aortic arch (mmHg) | 98 | 102 | 91 | 95 | 89 | 90 | 95 | |
| Mean ascending aorta flow (mL/min) | 5046 | 5017 | 4012 | 4007 | 3076 | 3038 | 1997 | 2039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reymond, P.; Bendjelid, K.; Giraud, R.; Richard, G.; Murith, N.; Cikirikcioglu, M.; Huber, C. To Balloon or Not to Balloon? The Effects of an Intra-Aortic Balloon-Pump on Coronary Artery Flow during Extracorporeal Circulation Simulating Normal and Low Cardiac Output Syndromes. J. Clin. Med. 2021, 10, 5333. https://doi.org/10.3390/jcm10225333
Reymond P, Bendjelid K, Giraud R, Richard G, Murith N, Cikirikcioglu M, Huber C. To Balloon or Not to Balloon? The Effects of an Intra-Aortic Balloon-Pump on Coronary Artery Flow during Extracorporeal Circulation Simulating Normal and Low Cardiac Output Syndromes. Journal of Clinical Medicine. 2021; 10(22):5333. https://doi.org/10.3390/jcm10225333
Chicago/Turabian StyleReymond, Philippe, Karim Bendjelid, Raphaël Giraud, Gérald Richard, Nicolas Murith, Mustafa Cikirikcioglu, and Christoph Huber. 2021. "To Balloon or Not to Balloon? The Effects of an Intra-Aortic Balloon-Pump on Coronary Artery Flow during Extracorporeal Circulation Simulating Normal and Low Cardiac Output Syndromes" Journal of Clinical Medicine 10, no. 22: 5333. https://doi.org/10.3390/jcm10225333