Developing a Prediction Model for 7-Year and 10-Year All-Cause Mortality Risk in Type 2 Diabetes Using a Hospital-Based Prospective Cohort Study
Abstract
:1. Introduction
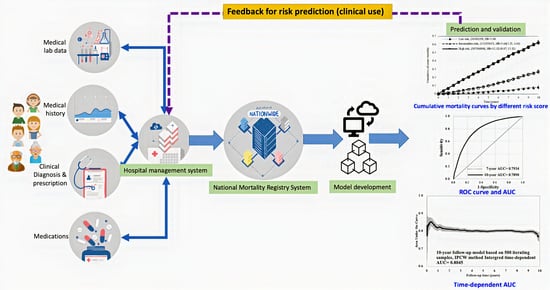
2. Materials and Methods
2.1. Study Design, Population, and Data Source
2.2. Definitions for Comorbidity and Biomarkers
2.3. Study Observational End Points
2.4. Statistical Analysis
2.5. Model Selection and Development
2.6. Model Performance
2.7. Model Validation
3. Results
3.1. Characteristic of Study Subjects
3.2. Factors and Coefficients of Prediction Models for All-Cause Mortality
- 7-year all-cause mortality risk score for individuals with type 2 diabetes
- = 0.3941 × aged 50–59 (if yes = 1) + 0.9882 × aged 60–69 (if yes = 1) + 1.7294 × aged ≥ 70 (if yes = 1)
- + 0.1867 × sex (if male = 1)
- + 0.3364 × history of cancer (if yes = 1)
- + 0.2615 × history of hypertension (if yes = 1)
- − 0.5407 × use of antihyperlipidemic drugs (if yes = 1)
- + 0.2440 × HbA1c (if abnormal = 1) + 0.2154 × HbA1c (if missing = 1)
- + 0.9154 × creatinine (if abnormal = 1) − 0.3088 × creatinine (if missing = 1)
- + 0.2569 × LDL /HDL ratio (if abnormal = 1) + 0.9216 × LDL /HDL ratio (if missing = 1)
- 10-year all-cause mortality risk score for individuals with type 2 diabetes
- = 0.3910 × aged 50–59 (if yes = 1) + 0.9908 × aged 60–69 (if yes = 1) +1.7198 × aged ≥ 70 (if yes = 1)
- + 0.2163 × sex (if male = 1)
- + 0.3860 × history of cancer (if yes = 1)
- + 0.3439 × history of hypertension (if yes = 1)
- − 0.4290 × use of antihyperlipidemic drugs (if yes = 1)
- + 0.2131 × HbA1c (if abnormal = 1) + 0.2126 × HbA1c (if missing =1)
- + 0.8793 × creatinine (if abnormal = 1) − 0.1934 × creatinine (if missing = 1)
- + 0.2056 × LDL /HDL ratio (if abnormal = 1) − 0.6472 × LDL /HDL ratio (if missing=1)
3.3. Performance of Prediction Models for All-Cause Mortality
3.4. Validation of Prediction Models for All-Cause Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20-79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, D.; Wen, W.; Saito, E.; Rahman, S.; Shu, X.O.; Chen, Y.; Gupta, P.C.; Gu, D.; Tsugane, S.; et al. Association of Diabetes With All-Cause and Cause-Specific Mortality in Asia: A Pooled Analysis of More Than 1 Million Participants. JAMA Netw. Open 2019, 2, e192696. [Google Scholar] [CrossRef] [PubMed]
- Yourman, L.C.; Lee, S.J.; Schonberg, M.A.; Widera, E.W.; Smith, A.K. Prognostic indices for older adults: A systematic review. JAMA 2012, 307, 182–192. [Google Scholar] [CrossRef] [PubMed]
- McEwen, L.N.; Kim, C.; Karter, A.J.; Haan, M.N.; Ghosh, D.; Lantz, P.M.; Mangione, C.M.; Thompson, T.J.; Herman, W.H. Risk factors for mortality among patients with diabetes: The Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care 2007, 30, 1736–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, L.N.; Karter, A.J.; Waitzfelder, B.E.; Crosson, J.C.; Marrero, D.G.; Mangione, C.M.; Herman, W.H. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD). Diabetes Care 2012, 35, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Rachel, L.J.F.; Chiang, T.L. Evolution of Taiwan’s health care system. Health Econ. Policy Law 2011, 6, 85–107. [Google Scholar] [CrossRef]
- Lu, J.R.; Chiang, T.L. Developing an adequate supply of health services: Taiwan’s path to Universal Health Coverage. Soc. Sci. Med. 2018, 198, 7–13. [Google Scholar] [CrossRef]
- Chou, C.W.; Kung, P.T.; Chou, W.Y.; Tsai, W.C. Pay-for-performance programmes reduce stroke risks in patients with type 2 diabetes: A national cohort study. BMJ Open 2019, 9, e026626. [Google Scholar] [CrossRef]
- Li, H.Y.; Wu, Y.L.; Tu, S.T.; Hwu, C.M.; Liu, J.S.; Chuang, L.M. Trends of mortality in diabetic patients in Taiwan: A nationwide survey in 2005-2014. J. Formos Med. Assoc. 2019, 118 (Suppl. 2), S83–S89. [Google Scholar] [CrossRef]
- Lin, J.C.; Shau, W.Y.; Lai, M.S. Sex- and age-specific prevalence and incidence rates of sight-threatening diabetic retinopathy in Taiwan. JAMA Ophthalmol. 2014, 132, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Groenwold, R.H.; White, I.R.; Donders, A.R.; Carpenter, J.R.; Altman, D.G.; Moons, K.G. Missing covariate data in clinical research: When and when not to use the missing-indicator method for analysis. CMAJ 2012, 184, 1265–1269. [Google Scholar] [CrossRef] [Green Version]
- Tibshirani, R. The lasso method for variable selection in the Cox model. Stat Med. 1997, 16, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; So, Y.; Jang, W. Evaluating Predictive Accuracy of Survival Models with PROC PHREG. In Proceedings of the SAS Global Forum 2017, Orlando, FL, USA, 2–5 April 2017. [Google Scholar]
- Gardiner, J.C. Evaluating the Accuracy of Clinical Prediction Models for Binary and Survival Outcomes; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]
- Blanche, P.; Dartigues, J.F.; Jacqmin-Gadda, H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat. Med. 2013, 32, 5381–5397. [Google Scholar] [CrossRef]
- Abdi, H. Bonferroni and Šidák corrections for multiple comparisons. In Encyclopedia of Measurement and Statistics; Salkind, N.J., Ed.; Sage: Thousand Oaks, CA, USA, 2007; Available online: https://personal.utdallas.edu/~herve/Abdi-Bonferroni2007-pretty.pdf (accessed on 17 October 2021).
- Robinson, T.E.; Elley, C.R.; Kenealy, T.; Drury, P.L. Development and validation of a predictive risk model for all-cause mortality in type 2 diabetes. Diabetes Res. Clin. Pract. 2015, 108, 482–488. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Fong, D.Y.T.; Fung, C.S.C.; Yu, E.Y.T.; Chin, W.Y.; Chan, A.K.C.; Lam, C.L.K. Prediction of five-year all-cause mortality in Chinese patients with type 2 diabetes mellitus—A population-based retrospective cohort study. J. Diabetes Complicat. 2017, 31, 939–944. [Google Scholar] [CrossRef]
- De Cosmo, S.; Copetti, M.; Lamacchia, O.; Fontana, A.; Massa, M.; Morini, E.; Pacilli, A.; Fariello, S.; Palena, A.; Rauseo, A.; et al. Development and validation of a predicting model of all-cause mortality in patients with type 2 diabetes. Diabetes Care. 2013, 36, 2830–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copetti, M.; Shah, H.; Fontana, A.; Scarale, M.G.; Menzaghi, C.; De Cosmo, S.; Garofolo, M.; Sorrentino, M.R.; Lamacchia, O.; Penno, G.; et al. Estimation of Mortality Risk in Type 2 Diabetic Patients (ENFORCE): An Inexpensive and Parsimonious Prediction Model. J. Clin. Endocrinol. Metab. 2019, 104, 4900–4908. [Google Scholar] [CrossRef] [Green Version]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong Kong Diabetes Registry; Yang, X.; So, W.Y.; Tong, P.C.; Ma, R.C.; Kong, A.P.; Lam, C.W.; Ho, C.S.; Cockram, C.S.; Ko, G.T.; et al. Development and validation of an all-cause mortality risk score in type 2 diabetes. Arch. Intern. Med. 2008, 168, 451–457. [Google Scholar]
- Taylor, F.; Ward, K.; Moore, T.H.; Burke, M.; Smith, G.D.; Casas, J.P.; Ebrahim, S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, CD004816. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Hayen, A.; Zhu, L.; McGeechan, K.; Glasziou, P.; Irwig, L.; Doust, J.; Gregory, G.; Bell, K. Legacy effects of statins on cardiovascular and all-cause mortality: A meta-analysis. BMJ Open 2018, 8, e020584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, C.S.C.; Wan, E.Y.F.; Chan, A.K.C.; Lam, C.L.K. Statin use reduces cardiovascular events and all-cause mortality amongst Chinese patients with type 2 diabetes mellitus: A 5-year cohort study. BMC Cardiovasc. Disord. 2017, 17, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.H.; Wang, J.S.; Lin, S.Y.; Li, C.H.; Wang, C.Y.; Hu, C.Y.; Fu, C.P.; Tsai, Y.T.; Lee, A.T.; Chen, Y.W.; et al. Effects of statins on all-cause mortality at different low-density-lipoprotein cholesterol levels in Asian patients with type 2 diabetes. Curr. Med. Res. Opin. 2018, 34, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Lin, C.H.; Yang, S.Y.; Chiang, J.H.; Lin, C.C. Development and validation of prediction models for the risks of diabetes-related hospitalization and in-hospital mortality in patients with type 2 diabetes. Metabolism 2018, 85, 38–47. [Google Scholar] [CrossRef]




| Variables | 7-Year Follow-Up | 10-Year Follow-Up | ||||
|---|---|---|---|---|---|---|
| No. Deaths | Person Years | Mortality Rate (per 100) (95% CI) | No. Deaths | Person Years | Mortality Rate (per 100) (95% CI) | |
| Overall | 2779 | 79,427.1 | 3.50 (2.20, 4.80) | 4561 | 12,2929.5 | 3.71 (2.63, 4.79) |
| Age at entry | ||||||
| <50 y/o | 169 | 16,277.6 | 1.04 (0.00, 2.61) | 307 | 26,328.9 | 1.17 (0.00, 2.47) |
| 50–59 y/o | 322 | 21,426.9 | 1.50 (0.00, 3.14) | 588 | 34,384.4 | 1.71 (0.33, 3.09) |
| 60–69 y/o | 601 | 19,729.5 | 3.05 (0.61, 5.49) | 1055 | 30,701.2 | 3.44 (1.37, 5.51) |
| ≥70 y/o | 1687 | 21,993.1 | 7.67 (4.01, 11.33) | 2611 | 31,515.0 | 8.28 (5.10, 11.46) |
| Sex | ||||||
| Female | 1337 | 40,035.8 | 3.34 (1.55, 5.13) | 2179 | 61,942.9 | 3.52 (2.04, 5.00) |
| Male | 1442 | 39,391.3 | 3.66 (1.77, 5.55) | 2382 | 60,986.6 | 3.91 (2.34, 5.48) |
| History of cancer | ||||||
| No | 1867 | 60,762.9 | 3.07 (1.68, 4.46) | 3075 | 95,153.6 | 3.23 (2.09, 4.37) |
| Yes | 912 | 18,664.2 | 4.89 (1.72, 8.06) | 1486 | 27,775.8 | 5.35 (2.63, 8.07) |
| History of PVD | ||||||
| No | 2635 | 76,777.0 | 3.43 (2.12, 4.74) | 4326 | 11,9076.3 | 3.63 (2.55, 4.71) |
| Yes | 144 | 2650.1 | 5.43 (0.0, 14.31) | 235 | 3853.2 | 6.10 (0.00, 13.90) |
| History of hypertension | ||||||
| No | 381 | 16,555.4 | 2.30 (0.0, 4.61) | 581 | 27,295.1 | 2.13 (0.40, 3.86) |
| Yes | 2398 | 62,871.7 | 3.81 (2.28, 5.34) | 3980 | 95,634.3 | 4.16 (2.87, 5.45) |
| Use of antihypertensive drugs | ||||||
| No | 648 | 23,583.9 | 2.75 (0.63, 4.87) | 967 | 38,361.7 | 2.52 (0.93, 4.11) |
| Yes | 2131 | 55,843.2 | 3.82 (2.20, 5.44) | 3594 | 84,567.8 | 4.25 (2.86, 5.64) |
| History of hyperlipidemia | ||||||
| No | 1478 | 23,427.9 | 6.31 (3.09, 9.53) | 2124 | 36,215.8 | 5.86 (3.37, 8.35) |
| Yes | 1301 | 55,999.2 | 2.32 (1.06, 3.58) | 2437 | 86,713.7 | 2.81 (1.69, 3.93) |
| Use of antihyperlipidemic drugs | ||||||
| No | 1685 | 33,496.2 | 5.03 (2.63, 7.43) | 2517 | 51,989.5 | 4.84 (2.95, 6.73) |
| Yes | 1094 | 45,930.9 | 2.38 (0.97, 3.79) | 2044 | 70,940.0 | 2.88 (1.63, 4.13) |
| HbA1c | ||||||
| Normal (<7) | 1034 | 33,460.8 | 3.09 (1.21, 4.97) | 1754 | 51,138.8 | 3.43 (1.82, 5.04) |
| Abnormal (≧7) | 1081 | 33,826.6 | 3.20 (1.29, 5.11) | 1838 | 51,850.1 | 3.54 (1.92, 5.16) |
| Missing | 664 | 12,139.8 | 5.47 (1.31, 9.63) | 969 | 19,940.6 | 4.86 (1.80, 7.92) |
| Creatinine | ||||||
| Normal | 1191 | 54,274.7 | 2.19 (0.94, 3.44) | 2084 | 84,441.7 | 2.47 (1.41, 3.53) |
| Abnormal | 1379 | 18,294.5 | 7.54 (3.56, 11.52) | 2107 | 26,179.9 | 8.05 (4.61, 11.49) |
| Missing | 209 | 68,57.9 | 3.05 (0.0, 7.18) | 370 | 12,307.9 | 3.01 (0.0, 6.07) |
| Total cholesterol | ||||||
| Normal (<200) | 1460 | 44,192.5 | 3.30 (1.61, 4.99) | 2406 | 67,844.4 | 3.55 (2.13, 4.97) |
| Abnormal (≧200) | 606 | 25,712.4 | 2.36 (0.48, 4.24) | 1140 | 39,098.5 | 2.92 (1.23, 4.61) |
| Missing | 713 | 9522.2 | 7.49 (1.99, 12.99) | 1015 | 15,986.6 | 6.35 (2.44, 10.26) |
| Triglyceride | ||||||
| Normal (<150) | 1392 | 44,884.8 | 3.10 (1.47, 4.73) | 2321 | 67,964.7 | 3.42 (2.03, 4.81) |
| Abnormal (≧150) | 626 | 24,692.3 | 2.54 (0.55, 4.53) | 1172 | 38,567.3 | 3.04 (1.30, 4.78) |
| Missing | 761 | 9850.0 | 7.73 (2.24, 13.22) | 1068 | 16,397.4 | 6.51 (2.60, 10.42) |
| LDL | ||||||
| Normal (<100) | 720 | 22,242.0 | 3.24 (0.88, 5.60) | 1253 | 34,843.8 | 3.60 (1.61, 5.59) |
| Abnormal (≧100) | 1143 | 44,533.7 | 2.57 (1.08, 4.06) | 2026 | 67,480.9 | 3.00 (1.69, 4.31) |
| Missing | 916 | 12,651.3 | 7.24 (2.55, 11.93) | 1282 | 20,604.8 | 6.22 (2.81, 9.63) |
| HDL | ||||||
| Normal | 424 | 17,769.8 | 2.39 (0.12, 4.66) | 782 | 28,603.1 | 2.73 (0.81, 4.65) |
| Abnormal | 1430 | 49,105.7 | 2.91 (1.40, 4.42) | 2483 | 73,860.2 | 3.36 (2.04, 4.68) |
| Missing | 925 | 12,551.7 | 7.37 (2.62, 12.12) | 1296 | 20,466.2 | 6.33 (2.88, 9.78) |
| LDL /HDL ratio | ||||||
| Normal | 928 | 37,921.2 | 2.45 (0.88, 4.02) | 1737 | 58,961.9 | 2.95 (1.56, 4.34) |
| Abnormal | 826 | 26,064.1 | 3.17 (1.01, 5.33) | 1368 | 37,433.1 | 3.65 (1.71, 5.59) |
| Missing | 1025 | 15,441.8 | 6.64 (2.58, 10.70) | 1456 | 26,534.5 | 5.49 (2.67, 8.31) |
| Variable | 10-Year Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| β | HR (95% CI) | β | aHR (95% CI) | β | HR (95% CI) | β | aHR (95% CI) | |
| Age at entry | ||||||||
| 50–59 vs. <50 y/o | 0.3698 | 1.45 (1.20, 1.74) | 0.3941 | 1.48 (1.23, 1.79) | 0.3838 | 1.47 (1.28, 1.69) | 0.3910 | 1.48 (1.29, 1.70) |
| 60–69 vs. <50 y/o | 1.0729 | 2.92 (2.47, 3.47) | 0.9882 | 2.69 (2.26, 3.19) | 1.0815 | 2.95 (2.60, 3.35) | 0.9908 | 2.69 (2.37, 3.06) |
| ≥70 vs. <50 y/o | 1.9937 | 7.34 (6.27, 8.60) | 1.7294 | 5.64 (4.79, 6.64) | 1.9628 | 7.12 (6.33, 8.01) | 1.7198 | 5.58 (4.94, 6.31) |
| Sex | ||||||||
| Male vs. Female | 0.0917 | 1.10 (1.02, 1.18) | 0.1867 | 1.21 (1.12, 1.30) | 0.1059 | 1.11 (1.05, 1.18) | 0.2163 | 1.24 (1.17, 1.32) |
| History of cancer | ||||||||
| Yes vs. No | 0.4638 | 1.59 (1.47, 1.72) | 0.3364 | 1.40 (1.29, 1.52) | 0.5027 | 1.65 (1.55, 1.76) | 0.3860 | 1.47 (1.38, 1.57) |
| History of hypertension | ||||||||
| Yes vs. No | 0.5123 | 1.67 (1.50, 1.86) | 0.2615 | 1.30 (1.15, 1.46) | 0.6702 | 1.96 (1.79, 2.13) | 0.3439 | 1.41 (1.28, 1.55) |
| Use of antihyperlipidemic drugs | ||||||||
| Yes vs. No | −0.7397 | 0.48 (0.44, 0.52) | −0.5407 | 0.58 (0.53, 0.64) | −0.5182 | 0.60 (0.56, 0.63) | −0.4290 | 0.65 (0.61, 0.70) |
| HbA1c | ||||||||
| ≧7 vs. <7 | 0.0307 | 1.03 (0.95, 1.12) | 0.2440 | 1.28 (1.17, 1.39) | 0.0316 | 1.03 (0.97, 1.10) | 0.2131 | 1.24 (1.16, 1.32) |
| Missing vs. <7 | 0.5524 | 1.74 (1.58, 1.92) | 0.2154 | 1.24 (1.10, 1.40) | 0.3463 | 1.41 (1.31, 1.53) | 0.2126 | 1.24 (1.12, 1.37) |
| Creatinine | ||||||||
| Abnormal vs. normal | 1.2279 | 3.41 (3.16, 3.74) | 0.9154 | 2.50 (2.31, 2.71) | 1.1820 | 3.26 (3.07, 3.47) | 0.8793 | 2.41 (2.26, 2.56) |
| Missing vs. normal | 0.3032 | 1.35 (1.17, 1.57) | −0.3088 | 0.73 (0.62, 0.87) | 0.2035 | 1.23 (1.10, 1.37) | −0.1934 | 0.82 (0.73, 0.94) |
| LDL /HDL ratio | ||||||||
| Abnormal vs. normal | 0.2725 | 1.31 (1.20, 1.44) | 0.2569 | 1.29 (1.18, 1.42) | 0.2177 | 1.24 (1.16, 1.34) | 0.2056 | 1.23 (1.14, 1.32) |
| Missing vs. normal | 0.9760 | 2.65 (2.43, 2.90) | 0.9216 | 2.51 (2.26, 2.80) | 0.6260 | 1.87 (1.74, 2.01) | 0.6472 | 1.91 (1.75, 2.08) |
| Harrell’s C-statistic | 0.7955 | (0.7873, 0.8037) | 0.7775 | (0.7708, 0.7842) | ||||
| Integrated time-dependent AUC | 0.8169 | 0.8085 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, S.Y.-H.; Chen, Y.I.; Lu, J.R.; Ng, S.-C.; Chen, C.-H. Developing a Prediction Model for 7-Year and 10-Year All-Cause Mortality Risk in Type 2 Diabetes Using a Hospital-Based Prospective Cohort Study. J. Clin. Med. 2021, 10, 4779. https://doi.org/10.3390/jcm10204779
Chiu SY-H, Chen YI, Lu JR, Ng S-C, Chen C-H. Developing a Prediction Model for 7-Year and 10-Year All-Cause Mortality Risk in Type 2 Diabetes Using a Hospital-Based Prospective Cohort Study. Journal of Clinical Medicine. 2021; 10(20):4779. https://doi.org/10.3390/jcm10204779
Chicago/Turabian StyleChiu, Sherry Yueh-Hsia, Ying Isabel Chen, Juifen Rachel Lu, Soh-Ching Ng, and Chih-Hung Chen. 2021. "Developing a Prediction Model for 7-Year and 10-Year All-Cause Mortality Risk in Type 2 Diabetes Using a Hospital-Based Prospective Cohort Study" Journal of Clinical Medicine 10, no. 20: 4779. https://doi.org/10.3390/jcm10204779