The Prognosis of Baseline Mitral Regurgitation in Patients with Transcatheter Aortic Valve Implantation
Abstract
:1. Introduction
2. Method
2.1. Patients and Data Collection
2.2. Statistical Analysis
2.3. Patient and Public Involvement
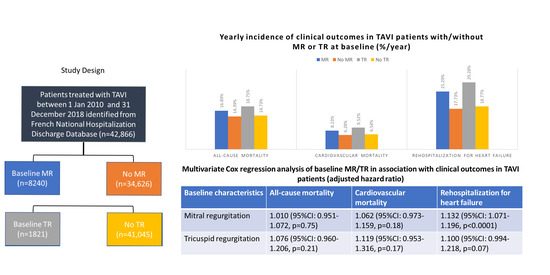
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakos, A.; Wilson-Smith, A.; Arora, S.; Nguyen, T.C.; Dhoble, A.; Tarantini, G.; Thielmann, M.; Vavalle, J.P.; Wendt, D.; Yan, T.D.; et al. Long term outcomes of transcatheter aortic valve implantation (TAVI): A systematic review of 5-year survival and beyond. Ann. Cardiothorac. Surg. 2017, 6, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Ludman, P.F.; Moat, N.; de Belder, M.A.; Blackman, D.J.; Duncan, A.; Banya, W.; MacCarthy, P.A.; Cunningham, D.; Wendler, O.; Marlee, D.; et al. Transcatheter aortic valve implantation in the United Kingdom: Temporal trends, predictors of outcome, and 6-year follow-up: A report from the UK Transcatheter Aortic Valve Implantation (TAVI) Registry, 2007 to 2012. Circulation 2015, 131, 1181–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overtchouk, P.; Guedeney, P.; Rouanet, S.; Verhoye, J.P.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Gilard, M.; Leprince, P.; Iung, B.; et al. Long-Term Mortality and Early Valve Dysfunction According to Anticoagulation Use: The FRANCE TAVI Registry. J. Am. Coll. Cardiol. 2019, 73, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Mavromatis, K.; Thourani, V.H.; Stebbins, A.; Vemulapalli, S.; Devireddy, C.; Guyton, R.A.; Matsouaka, R.; Ghasemzadeh, N.; Block, P.C.; Leshnower, B.G.; et al. Transcatheter Aortic Valve Replacement in Patients With Aortic Stenosis and Mitral Regurgitation. Ann. Thorac. Surg. 2017, 104, 1977–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenbroich, R.; Stortecky, S.; Praz, F.; Lanz, J.; Franzone, A.; Zuk, K.; Heg, D.; Valgimigli, M.; O’Sullivan, C.J.; Heinisch, C.; et al. The impact of functional vs degenerative mitral regurgitation on clinical outcomes among patients undergoing transcatheter aortic valve implantation. Am. Heart. J. 2017, 184, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Amat-Santos, I.J.; Nombela-Franco, L.; Munoz-Garcia, A.J.; Gutierrez-Ibanes, E.; De La Torre Hernandez, J.M.; Cordoba-Soriano, J.G.; Jimenez-Quevedo, P.; Hernandez-Garcia, J.M.; Gonzalez-Mansilla, A.; et al. Mitral Regurgitation After Transcatheter Aortic Valve Replacement: Prognosis, Imaging Predictors, and Potential Management. JACC Cardiovasc. Interv. 2016, 9, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, F.; Latib, A.; De Marco, F.; Agnifili, M.; Oreglia, J.; Pizzocri, S.; Latini, R.A.; Lanotte, S.; Petronio, A.S.; De Carlo, M.; et al. Interplay between mitral regurgitation and transcatheter aortic valve replacement with the CoreValve Revalving System: A multicenter registry. Circulation 2013, 128, 2145–2153. [Google Scholar] [CrossRef] [Green Version]
- Boerlage-van Dijk, K.; Wiegerinck, E.M.; Takama, T.; Koch, K.T.; Vis, M.M.; de Mol, B.A.; Piek, J.J.; Bouma, B.J.; Baan, J., Jr. Mitral regurgitation prior to transcatheter aortic valve implantation influences survival but not symptoms. Int. J. Cardiol. 2016, 204, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Wind, A.M.; Cramer, M.J.; Nijhoff, F.; Agostoni, P.; Ramjankhan, F.Z.; Suyker, W.J.; Stella, P.R.; Chamuleau, S.A.J. Evaluation of mitral regurgitation by an integrated 2D echocardiographic approach in patients undergoing transcatheter aortic valve replacement. Int. J. Cardiovasc. Imaging 2018, 34, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Kiramijyan, S.; Magalhaes, M.A.; Koifman, E.; Didier, R.; Escarcega, R.O.; Minha, S.; Baker, N.C.; Negi, S.I.; Torguson, R.; Gai, J.; et al. Impact of baseline mitral regurgitation on short- and long-term outcomes following transcatheter aortic valve replacement. Am. Heart J. 2016, 178, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Silberman, S.; Fink, D.; Butnaru, A.; Balkin, J.; Almagor, Y.; Tauber, R.; Merin, O. Untreated Mitral Regurgitation Does Not Affect Survival of Elderly Patients Undergoing TAVI. J. Heart Valve Dis. 2016, 25, 46–50. [Google Scholar]
- Hutter, A.; Bleiziffer, S.; Richter, V.; Opitz, A.; Hettich, I.; Mazzitelli, D.; Ruge, H.; Lange, R. Transcatheter aortic valve implantation in patients with concomitant mitral and tricuspid regurgitation. Ann. Thorac. Surg. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Eltchaninoff, H.; Zahn, R.; Testa, L.; León, M.B.; Trillo-Nouche, R.; Augusto, D.; Smith, C.R.; Webb, J.; Bleiziffer, S.; et al. Clinical impact and evolution of mitral regurgitation following transcatheter aortic valve replacement: A meta-analysis. Heart 2015, 101, 1395–1405. [Google Scholar] [CrossRef]
- Lindman, B.R.; Maniar, H.S.; Jaber, W.A.; Lerakis, S.; Mack, M.J.; Suri, R.M.; Thourani, V.H.; Babaliaros, V.; Kereiakes, D.J.; Whisenant, B.; et al. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: Insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ. Cardiovasc. Interv. 2015, 8, e002073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.A.; Rozenbaum, Z.; Ghantous, E.; Kramarz, J.; Biner, S.; Ghermezi, M.; Shimiaie, J.; Finkelstein, A.; Banai, S.; Aviram, G.; et al. Impact of Right Ventricular Dysfunction and Tricuspid Regurgitation on Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2017, 30, 36–46. [Google Scholar] [CrossRef]
- Khan, F.; Okuno, T.; Malebranche, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Transcatheter Aortic Valve Replacement in Patients With Multivalvular Heart Disease. JACC Cardiovasc. Interv. 2020, 13, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, X.; Yu, L.; Sun, Y.; Jaiswal, S.; Zhu, Q.; Chen, H.; He, Y.; Wang, L.; Ren, K.; et al. Impact of tricuspid regurgitation and right ventricular dysfunction on outcomes after transcatheter aortic valve replacement: A systematic review and meta-analysis. Clin. Cardiol. 2019, 42, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Etienne, C.S.; Grammatico-Guillon, L.; Porto, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Impact of Sapien 3 Balloon-Expandable Versus Evolut R Self-Expandable Transcatheter Aortic Valve Implantation in Patients With Aortic Stenosis: Data From a Nationwide Analysis. Circulation 2020, 141, 260–268. [Google Scholar] [CrossRef]
- Fukui, M.; Gupta, A.; Abdelkarim, I.; Sharbaugh, M.S.; Althouse, A.D.; Elzomor, H.; Mulukutla, S.; Lee, J.S.; Schindler, J.T.; Gleason, T.G.; et al. Association of Structural and Functional Cardiac Changes With Transcatheter Aortic Valve Replacement Outcomes in Patients With Aortic Stenosis. JAMA Cardiol. 2019, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Feldt, K.; De Palma, R.; Bjursten, H.; Petursson, P.; Nielsen, N.E.; Kellerth, T.; Jonsson, A.; Nilsson, J.; Ruck, A.; Settergren, M. Change in mitral regurgitation severity impacts survival after transcatheter aortic valve replacement. Int. J. Cardiol. 2019, 294, 32–36. [Google Scholar] [CrossRef]
- Zahn, R.; Gerckens, U.; Linke, A.; Sievert, H.; Kahlert, P.; Hambrecht, R.; Sack, S.; Abdel-Wahab, M.; Hoffmann, E.; Schiele, R.; et al. Predictors of one-year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am. J. Cardiol. 2013, 112, 272–279. [Google Scholar] [CrossRef]
- Wendler, O.; Schymik, G.; Treede, H.; Baumgartner, H.; Dumonteil, N.; Neumann, F.J.; Tarantini, G.; Zamorano, J.L.; Vahanian, A. SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur. Heart J. 2017, 38, 2717–2726. [Google Scholar] [CrossRef] [Green Version]
- Schymik, G.; Lefevre, T.; Bartorelli, A.L.; Rubino, P.; Treede, H.; Walther, T.; Baumgartner, H.; Windecker, S.; Wendler, O.; Urban, P.; et al. European experience with the second-generation Edwards SAPIEN XT transcatheter heart valve in patients with severe aortic stenosis: 1-year outcomes from the SOURCE XT Registry. JACC Cardiovasc. Interv. 2015, 8, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Ben-Assa, E.; Biner, S.; Banai, S.; Arbel, Y.; Laufer-Perl, M.; Kramarz, J.; Elmariah, S.; Inglessis, I.; Keren, G.; Finkelstein, A.; et al. Clinical impact of post procedural mitral regurgitation after transcatheter aortic valve replacement. Int. J. Cardiol. 2020, 299, 215–221. [Google Scholar] [CrossRef]
- Hermiller, J.B., Jr.; Yakubov, S.J.; Reardon, M.J.; Deeb, G.M.; Adams, D.H.; Afilalo, J.; Huang, J.; Popma, J.J.; CoreValve United States Clinical Investigators. Predicting Early and Late Mortality After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 68, 343–352. [Google Scholar] [CrossRef]
- Edwards, F.H.; Cohen, D.J.; O’Brien, S.M.; Peterson, E.D.; Mack, M.J.; Shahian, D.M.; Grover, F.L.; Tuzcu, E.M.; Thourani, V.H.; Carroll, J.; et al. Development and Validation of a Risk Prediction Model for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2016, 1, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, V.; Schirmer, J.; Franzen, O.; Schluter, M.; Seiffert, M.; Treede, H.; Reichenspurner, H.; Blankenberg, S.; Baldus, S. Bivalvular transcatheter treatment of high-surgical-risk patients with coexisting severe aortic stenosis and significant mitral regurgitation. Int. J. Cardiol. 2013, 167, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Stahli, B.E.; Reinthaler, M.; Leistner, D.M.; Landmesser, U.; Lauten, A. Transcatheter Aortic Valve Replacement and Concomitant Mitral Regurgitation. Front. Cardiovasc. Med. 2018, 5, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linke, A. Treatment of Concomitant Mitral Regurgitation by Mitral Valve Clipping in Patients With Successful Transcatheter Aortic Valve Implantation (MITAVI). Available online: https://clinicaltrials.gov/ct2/show/NCT04009434 (accessed on 13 August 2021).
- Amat-Santos, I.J.; Castrodeza, J.; Nombela-Franco, L.; Munoz-Garcia, A.J.; Gutierrez-Ibanes, E.; Hernandez, J.M.d.l.T.; Cordoba-Soriano, J.G.; Jimenez-Quevedo, P.; Hernandez-Garcia, J.M.; Gonzalez-Mansilla, A.; et al. Tricuspid but not Mitral Regurgitation Determines Mortality After TAVI in Patients With Nonsevere Mitral Regurgitation. Rev. Esp. Cardiol. 2018, 71, 357–364. [Google Scholar] [CrossRef]
- Gotzmann, M.; Pljakic, A.; Bojara, W.; Lindstaedt, M.; Ewers, A.; Germing, A.; Mugge, A. Transcatheter aortic valve implantation in patients with severe symptomatic aortic valve stenosis-predictors of mortality and poor treatment response. Am. Heart J. 2011, 162, 238–245. [Google Scholar] [CrossRef]
- Tarantini, G.; Baumgartner, H.; Frank, D.; Husser, O.; Bleiziffer, S.; Rudolph, T.; Jeger, R.; Fraccaro, C.; Hovorka, T.; Wendler, O. Four-year mortality in women and men after transfemoral transcatheter aortic valve implantation using the SAPIEN. Catheter. Cardiovasc. Interv. 2021, 97, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kiyohara, K.; Sato, D.; Kitamura, T.; Kodera, S. A Real-World Comparison of 1-Year Survival and Expenditures for Transcatheter Aortic Valve Replacements: SAPIEN 3 Versus CoreValve Versus Evolut R. Value Health 2021, 24, 497–504. [Google Scholar] [CrossRef]
- D’Auria, F.; Casado, A.P.; Myat, A.; Lorusso, R.; Hildick-Smith, D. Long-Term Survival and Outcomes According to VARC-2 Criteria for Subclavian, Direct Aortic, Femoral, and Apical Implantation: An 8-Year United Kingdom TAVI Surgical Experience. Surg. Technol. Int. 2020, 37, 245–252. [Google Scholar]
- Wessler, B.S.; Weintraub, A.R.; Udelson, J.E.; Kent, D.M. Can Clinical Predictive Models Identify Patients Who Should Not Receive TAVR? A Systematic Review. Struct. Heart 2020, 4, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Korber, M.I.; Kuhn, E.; Schmidt, T.; Frerker, C.; Wahlers, T.; Rudolph, T.K.; Baldus, S.; Adam, M.; Ten Freyhaus, H. Prognosis of persistent mitral regurgitation in patients undergoing transcatheter aortic valve replacement. Clin. Res. Cardiol. 2020, 109, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, F.H.; Vemulapalli, S.; Li, Z.; Thourani, V.; Matsouaka, R.A.; Desai, N.D.; Kirtane, A.; Anwaruddin, S.; Williams, M.L.; Giri, J.; et al. Association of Tricuspid Regurgitation With Transcatheter Aortic Valve Replacement Outcomes: A Report From The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann. Thorac. Surg. 2018, 105, 1121–1128. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, J.; Ikenaga, H.; Hayashi, A.; Yamaguchi, S.; Nagaura, T.; Rader, F.; Siegel, R.J.; Makkar, R.R.; Shiota, T. Predictors and Outcomes of Persistent Tricuspid Regurgitation After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2019, 124, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.P.; Bartko, P.; Hofer, F.; Zbiral, M.; Burger, A.; Ghanim, B.; Kastner, J.; Lang, I.M.; Mascherbauer, J.; Hengstenberg, C.; et al. Evolution of outcome and complications in TAVR: A meta-analysis of observational and randomized studies. Sci. Rep. 2020, 10, 15568. [Google Scholar] [CrossRef]



| No Mitral Regurgitation | Mitral Regurgitation | p | Total | |
|---|---|---|---|---|
| (n = 34,626) | (n = 8240) | (n = 42,866) | ||
| Age, years | 82.7 ± 6.7 | 82.7 ± 7.0 | 0.5 | 82.7 ± 6.8 |
| Sex (female) | 17,587 (50.8) | 4382 (53.2) | 0.0001 | 21,969 (51.3) |
| Charlson comorbidity index | 3.9 ± 2.8 | 4.6 ± 2.8 | <0.0001 | 4.0 ± 2.8 |
| Frailty index | 5.2 ± 5.7 | 6.4 ± 6.0 | <0.0001 | 5.4 ± 5.8 |
| EuroSCORE II | 3.6 ± 0.9 | 4.0 ± 1.0 | <0.0001 | 3.7 ± 1.0 |
| Hypertension | 27,618 (79.8) | 6918 (84.0) | <0.0001 | 34,536 (80.6) |
| Diabetes mellitus | 10,157 (29.3) | 2439 (29.6) | 0.63 | 12,596 (29.4) |
| Heart failure with congestion | 18,312 (52.9) | 5871 (71.3) | <0.0001 | 24,183 (56.4) |
| History of pulmonary oedema | 1591 (4.6) | 658 (8.0) | <0.0001 | 2249 (5.2) |
| Aortic regurgitation | 3162 (9.1) | 1975 (24.0) | <0.0001 | 5137 (12.0) |
| Mitral regurgitation | 0 (0.0) | 8240 (100.0) | - | 8240 (19.2) |
| Previous endocarditis | 189 (0.5) | 108 (1.3) | <0.0001 | 297 (0.7) |
| Dilated cardiomyopathy | 4839 (14.0) | 2064 (25.0) | <0.0001 | 6903 (16.1) |
| Coronary artery disease | 20,760 (60.0) | 5593 (67.9) | <0.0001 | 26,353 (61.5) |
| Previous myocardial infarction | 4618 (13.3) | 1460 (17.7) | <0.0001 | 6078 (14.2) |
| Previous PCI | 9834 (28.4) | 2599 (31.5) | <0.0001 | 12,433 (29.0) |
| Previous CABG | 2788 (8.1) | 839 (10.2) | <0.0001 | 3627 (8.5) |
| Vascular disease | 12,001 (34.7) | 3653 (44.3) | <0.0001 | 15,654 (36.5) |
| Atrial fibrillation | 14,684 (42.4) | 4691 (56.9) | <0.0001 | 19,375 (45.2) |
| Previous pacemaker or ICD | 6744 (19.5) | 2085 (25.3) | <0.0001 | 8829 (20.6) |
| Ischemic stroke | 1837 (5.3) | 492 (6.0) | 0.02 | 2329 (5.4) |
| Intracranial bleeding | 482 (1.4) | 154 (1.9) | 0.001 | 636 (1.5) |
| Smoker | 2512 (7.3) | 836 (10.1) | <0.0001 | 3348 (7.8) |
| Dyslipidemia | 15,793 (45.6) | 4237 (51.4) | <0.0001 | 20,030 (46.7) |
| Obesity | 8638 (24.9) | 2257 (27.4) | <0.0001 | 10,895 (25.4) |
| Alcohol related diagnoses | 1439 (4.2) | 380 (4.6) | 0.07 | 1819 (4.2) |
| Abnormal renal function | 5482 (15.8) | 2004 (24.3) | <0.0001 | 7486 (17.5) |
| Lung disease | 7888 (22.8) | 2274 (27.6) | <0.0001 | 10,162 (23.7) |
| Sleep apnea syndrome | 3030 (8.8) | 778 (9.4) | 0.05 | 3808 (8.9) |
| COPD | 5002 (14.4) | 1423 (17.3) | <0.0001 | 6425 (15.0) |
| Liver disease | 1552 (4.5) | 556 (6.7) | <0.0001 | 2108 (4.9) |
| Gastroesophageal reflux | 1126 (3.3) | 333 (4.0) | 0.0004 | 1459 (3.4) |
| Thyroid diseases | 4487 (13.0) | 1469 (17.8) | <0.0001 | 5956 (13.9) |
| Inflammatory disease | 3268 (9.4) | 1091 (13.2) | <0.0001 | 4359 (10.2) |
| Anemia | 9006 (26.0) | 2822 (34.2) | <0.0001 | 11,828 (27.6) |
| Previous cancer | 6454 (18.6) | 1558 (18.9) | 0.57 | 8012 (18.7) |
| Edwards Sapien XTTM | 3425 (9.9) | 807 (9.8) | 0.79 | 4232 (9.9) |
| Edwards Sapien 3 | 17,552 (50.7) | 4090 (49.6) | 0.09 | 21,642 (50.5) |
| Medtronic Corevalve | 4229 (12.2) | 1046 (12.7) | 0.23 | 5275 (12.3) |
| Medtronic Evolut | 9420 (27.2) | 2297 (27.9) | 0.22 | 11,717 (27.3) |
| Self-expandable TAVI | 13,649 (39.4) | 3343 (40.6) | 0.05 | 16,992 (39.6) |
| Balloon-expandable TAVI | 20,977 (60.6) | 4897 (59.4) | 0.05 | 25,874 (60.4) |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR, 95%CI | p | HR, 95%CI | p | |
| Age, years | 1.005 (1.002–1.009) | 0.002 | 1.016 (1.012–1.020) | <0.0001 |
| Charlson comorbidity index | 1.110 (1.101–1.118) | <0.0001 | 1.090 (1.077–1.103) | <0.0001 |
| Frailty index | 1.165 (1.136–1.195) | <0.0001 | 0.936 (0.907–0.965) | <0.0001 |
| Sex (male) | 1.218 (1.163–1.277) | <0.0001 | 1.146 (1.088–1.207) | <0.0001 |
| Hypertension | 1.046 (0.972–1.126) | 0.23 | 0.903 (0.836–0.976) | 0.01 |
| Diabetes mellitus | 1.115 (1.062–1.170) | <0.0001 | 0.906 (0.856–0.959) | 0.001 |
| Heart failure with congestion | 1.761 (1.671–1.855) | <0.0001 | 1.319 (1.246–1.398) | <0.0001 |
| History of pulmonary edema | 2.544 (2.365–2.735) | <0.0001 | 1.948 (1.805–2.102) | <0.0001 |
| Aortic regurgitation | 1.081 (1.010–1.156) | 0.02 | 0.967 (0.902–1.036) | 0.34 |
| Mitral regurgitation | 1.181 (1.116–1.249) | <0.0001 | 1.010 (0.951–1.072) | 0.75 |
| Tricuspid regurgitation | 1.272 (1.140–1.419) | <0.0001 | 1.076 (0.960–1.206) | 0.21 |
| Coronary artery disease | 1.092 (1.038–1.149) | 0.001 | 0.933 (0.878–0.990) | 0.02 |
| Previous myocardial infarction | 1.288 (1.211–1.369) | <0.0001 | 1.009 (0.935–1.088) | 0.82 |
| Previous PCI | 1.133 (1.077–1.192) | <0.0001 | 1.065 (1.005–1.128) | 0.03 |
| Previous CABG | 0.948 (0.876–1.027) | 0.19 | 0.953 (0.876–1.037) | 0.26 |
| Vascular disease | 1.296 (1.236–1.359) | <0.0001 | 1.075 (1.014–1.140) | 0.02 |
| Atrial fibrillation | 1.585 (1.511–1.661) | <0.0001 | 1.341 (1.276–1.409) | <0.0001 |
| Previous pacemaker or ICD | 1.182 (1.119–1.248) | <0.0001 | 0.997 (0.943–1.054) | 0.92 |
| Ischemic stroke | 1.255 (1.138–1.384) | <0.0001 | 1.050 (0.950–1.160) | 0.34 |
| Smoker | 1.194 (1.107–1.287) | <0.0001 | 1.013 (0.935–1.098) | 0.75 |
| Dyslipidemia | 0.917 (0.875–0.961) | <0.0001 | 0.857 (0.815–0.902) | <0.0001 |
| Obesity | 0.989 (0.940–1.041) | 0.68 | 0.919 (0.869–0.972) | 0.003 |
| Alcohol related diagnoses | 1.200 (1.095–1.316) | <0.0001 | 0.941 (0.849–1.042) | 0.24 |
| Abnormal renal function | 1.471 (1.393–1.553) | <0.0001 | 1.054 (0.994–1.117) | 0.08 |
| Lung disease | 1.408 (1.339–1.479) | <0.0001 | 1.173 (1.112–1.238) | <0.0001 |
| Sleep apnea syndrome | 1.242 (1.151–1.340) | <0.0001 | 1.099 (1.012–1.192) | 0.03 |
| Liver disease | 1.583 (1.452–1.726) | <0.0001 | 1.164 (1.056–1.283) | 0.002 |
| Thyroid diseases | 1.098 (1.028–1.173) | 0.005 | 0.969 (0.905–1.037) | 0.36 |
| Inflammatory disease | 1.295 (1.207–1.390) | <0.0001 | 1.131 (1.052–1.216) | 0.001 |
| Anemia | 1.458 (1.389–1.531) | <0.0001 | 1.176 (1.116–1.238) | <0.0001 |
| Previous cancer | 1.375 (1.301–1.453) | <0.0001 | 1.059 (0.995–1.126) | 0.07 |
| Edwards Sapien XT | 0.869 (0.817–0.923) | <0.0001 | 0.783 (0.721–0.850) | <0.0001 |
| Edwards Sapien 3 | 0.998 (0.949–1.049) | 0.93 | 0.875 (0.817–0.937) | <0.0001 |
| Medtronic Corevalve | 1.039 (0.982–1.099) | 0.18 | 0.839 (0.776–0.907) | <0.0001 |
| Medtronic Evolut | 1.124 (1.055–1.198) | <0.0001 | 1.000 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR, 95%CI | p | HR, 95%CI | p | |
| Age, years | 1.011 (1.005–1.016) | <0.0001 | 1.020 (1.014–1.026) | <0.0001 |
| Charlson comorbidity index | 1.069 (1.057–1.082) | <0.0001 | 1.014 (0.995–1.034) | 0.16 |
| Frailty index | 1.088 (1.048–1.130) | <0.0001 | 0.862 (0.822–0.903) | <0.0001 |
| Sex (male) | 1.041 (0.971–1.116) | 0.26 | 0.955 (0.884–1.032) | 0.25 |
| Hypertension | 0.982 (0.884–1.090) | 0.73 | 0.835 (0.747–0.933) | 0.001 |
| Diabetes mellitus | 1.101 (1.023–1.184) | 0.01 | 1.032 (0.947–1.124) | 0.48 |
| Heart failure with congestion | 2.142 (1.975–2.323) | <0.0001 | 1.661 (1.517–1.819) | <0.0001 |
| History of pulmonary edema | 3.631 (3.301–3.994) | <0.0001 | 2.732 (2.470–3.021) | <0.0001 |
| Aortic regurgitation | 1.150 (1.042–1.270) | 0.006 | 0.982 (0.887–1.088) | 0.73 |
| Mitral regurgitation | 1.313 (1.210–1.425) | <0.0001 | 1.062 (0.973–1.159) | 0.18 |
| Tricuspid regurgitation | 1.396 (1.196–1.630) | <0.0001 | 1.119 (0.953–1.316) | 0.17 |
| Coronary artery disease | 1.134 (1.051–1.224) | 0.001 | 0.918 (0.838–1.005) | 0.06 |
| Previous myocardial infarction | 1.473 (1.350–1.608) | <0.0001 | 1.055 (0.946–1.176) | 0.34 |
| Previous PCI | 1.208 (1.121–1.302) | <0.0001 | 1.110 (1.018–1.210) | 0.02 |
| Previous CABG | 1.120 (1.000–1.254) | 0.05 | 1.098 (0.973–1.240) | 0.13 |
| Vascular disease | 1.439 (1.342–1.544) | <0.0001 | 1.255 (1.149–1.369) | <0.0001 |
| Atrial fibrillation | 1.624 (1.513–1.743) | <0.0001 | 1.332 (1.237–1.435) | <0.0001 |
| Previous pacemaker or ICD | 1.219 (1.124–1.322) | <0.0001 | 1.007 (0.927–1.094) | 0.87 |
| Ischemic stroke | 1.440 (1.257–1.648) | <0.0001 | 1.372 (1.193–1.576) | <0.0001 |
| Smoker | 1.070 (0.952–1.204) | 0.26 | 0.970 (0.856–1.099) | 0.63 |
| Dyslipidemia | 0.975 (0.909–1.046) | 0.48 | 0.919 (0.851–0.991) | 0.03 |
| Obesity | 0.948 (0.878–1.024) | 0.18 | 0.892 (0.818–0.971) | 0.009 |
| Alcohol related diagnoses | 0.910 (0.779–1.064) | 0.24 | 0.799 (0.674–0.948) | 0.01 |
| Abnormal renal function | 1.583 (1.462–1.714) | <0.0001 | 1.212 (1.110–1.323) | <0.0001 |
| Lung disease | 1.413 (1.312–1.523) | <0.0001 | 1.266 (1.168–1.373) | <0.0001 |
| Sleep apnea syndrome | 1.290 (1.154–1.442) | <0.0001 | 1.220 (1.081–1.377) | 0.001 |
| Liver disease | 1.532 (1.343–1.747) | <0.0001 | 1.390 (1.203–1.608) | <0.0001 |
| Thyroid diseases | 1.099 (0.996–1.213) | 0.06 | 0.925 (0.835–1.024) | 0.13 |
| Inflammatory disease | 1.277 (1.149–1.419) | <0.0001 | 1.181 (1.060–1.317) | 0.003 |
| Anemia | 1.382 (1.285–1.487) | <0.0001 | 1.125 (1.041–1.216) | 0.003 |
| Previous cancer | 1.072 (0.981–1.171) | 0.12 | 0.990 (0.897–1.093) | 0.85 |
| Edwards Sapien XT | 0.981 (0.893–1.077) | 0.68 | 0.983 (0.872–1.109) | 0.79 |
| Edwards Sapien 3 | 0.843 (0.783–0.907) | <0.0001 | 0.875 (0.793–0.966) | 0.008 |
| Medtronic Corevalve | 1.225 (1.126–1.332) | <0.0001 | 1.067 (0.953–1.195) | 0.26 |
| Medtronic Evolut | 1.057 (0.966–1.158) | 0.23 | 1.000 | - |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| HR, 95%CI | p | HR, 95%CI | p | |
| Age, years | 1.014 (1.010–1.017) | <0.0001 | 1.021 (1.017–1.025) | <0.0001 |
| Charlson comorbidity index | 1.109 (1.101–1.117) | <0.0001 | 1.047 (1.035–1.059) | <0.0001 |
| Frailty index | 1.468 (1.433–1.503) | <0.0001 | 1.185 (1.151–1.220) | <0.0001 |
| Sex (male) | 0.982 (0.939–1.027) | 0.42 | 0.961 (0.915–1.010) | 0.12 |
| Hypertension | 1.755 (1.613–1.909) | <0.0001 | 1.283 (1.175–1.400) | <0.0001 |
| Diabetes mellitus | 1.381 (1.319–1.445) | <0.0001 | 1.109 (1.052–1.170) | <0.0001 |
| Heart failure with congestion | 2.614 (2.479–2.757) | <0.0001 | 1.828 (1.726–1.936) | <0.0001 |
| History of pulmonary edema | 1.631 (1.496–1.778) | <0.0001 | 1.075 (0.984–1.174) | 0.11 |
| Aortic regurgitation | 1.147 (1.076–1.222) | <0.0001 | 0.975 (0.913–1.040) | 0.44 |
| Mitral regurgitation | 1.411 (1.340–1.486) | <0.0001 | 1.132 (1.071–1.196) | <0.0001 |
| Tricuspid regurgitation | 1.509 (1.369–1.663) | <0.0001 | 1.100 (0.994–1.218) | 0.07 |
| Coronary artery disease | 1.334 (1.269–1.403) | <0.0001 | 1.143 (1.079–1.211) | <0.0001 |
| Previous myocardial infarction | 1.385 (1.308–1.468) | <0.0001 | 1.095 (1.019–1.177) | 0.01 |
| Previous PCI | 1.105 (1.052–1.160) | <0.0001 | 0.963 (0.912–1.017) | 0.18 |
| Previous CABG | 1.149 (1.070–1.233) | <0.0001 | 1.126 (1.043–1.215) | 0.002 |
| Vascular disease | 1.241 (1.187–1.299) | <0.0001 | 0.913 (0.862–0.966) | 0.002 |
| Atrial fibrillation | 2.078 (1.985–2.176) | <0.0001 | 1.630 (1.554–1.709) | <0.0001 |
| Previous pacemaker or ICD | 1.482 (1.410–1.558) | <0.0001 | 1.197 (1.138–1.260) | <0.0001 |
| Ischemic stroke | 1.126 (1.022–1.241) | 0.02 | 0.862 (0.781–0.952) | 0.003 |
| Smoker | 1.122 (1.042–1.208) | 0.002 | 1.014 (0.937–1.097) | 0.73 |
| Dyslipidemia | 1.150 (1.100–1.203) | <0.0001 | 1.003 (0.956–1.052) | 0.90 |
| Obesity | 1.326 (1.265–1.389) | <0.0001 | 1.112 (1.056–1.171) | <0.0001 |
| Alcohol related diagnoses | 1.080 (0.985–1.184) | 0.10 | 0.990 (0.895–1.095) | 0.84 |
| Abnormal renal function | 1.544 (1.466–1.626) | <0.0001 | 1.063 (1.006–1.124) | 0.03 |
| Lung disease | 1.393 (1.327–1.461) | <0.0001 | 1.149 (1.091–1.210) | <0.0001 |
| Sleep apnea syndrome | 1.343 (1.251–1.441) | <0.0001 | 1.048 (0.971–1.131) | 0.23 |
| Liver disease | 1.176 (1.069–1.293) | 0.001 | 0.938 (0.846–1.041) | 0.23 |
| Thyroid diseases | 1.373 (1.295–1.457) | <0.0001 | 1.062 (0.999–1.129) | 0.05 |
| Inflammatory disease | 1.256 (1.173–1.344) | <0.0001 | 0.994 (0.928–1.066) | 0.88 |
| Anemia | 1.302 (1.242–1.365) | <0.0001 | 0.992 (0.944–1.043) | 0.76 |
| Previous cancer | 0.957 (0.903–1.014) | 0.14 | 0.864 (0.810–0.920) | <0.0001 |
| Edwards Sapien XT | 0.799 (0.752–0.850) | <0.0001 | 0.609 (0.564–0.658) | <0.0001 |
| Edwards Sapien 3 | 0.950 (0.907–0.995) | 0.03 | 0.773 (0.728–0.822) | <0.0001 |
| Medtronic Corevalve | 1.008 (0.954–1.065) | 0.78 | 0.702 (0.654–0.755) | <0.0001 |
| Medtronic Evolut | 1.331 (1.259–1.408) | <0.0001 | 1.000 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bisson, A.; Boumhidi, J.; Herbert, J.; Saint Etienne, C.; Bernard, A.; Lip, G.Y.H.; Fauchier, L. The Prognosis of Baseline Mitral Regurgitation in Patients with Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 3974. https://doi.org/10.3390/jcm10173974
Zhang J, Bisson A, Boumhidi J, Herbert J, Saint Etienne C, Bernard A, Lip GYH, Fauchier L. The Prognosis of Baseline Mitral Regurgitation in Patients with Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2021; 10(17):3974. https://doi.org/10.3390/jcm10173974
Chicago/Turabian StyleZhang, Juqian, Arnaud Bisson, Jad Boumhidi, Julien Herbert, Christophe Saint Etienne, Anne Bernard, Gregory Y.H. Lip, and Laurent Fauchier. 2021. "The Prognosis of Baseline Mitral Regurgitation in Patients with Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 10, no. 17: 3974. https://doi.org/10.3390/jcm10173974